Muscular, skeletal, and neural adaptations following spinal cord injury
- PMID: 11838582
- PMCID: PMC4693289
- DOI: 10.2519/jospt.2002.32.2.65
Muscular, skeletal, and neural adaptations following spinal cord injury
Abstract
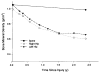
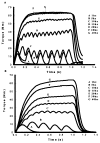
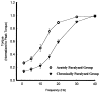
Spinal cord injury is associated with adaptations to the muscular, skeletal, and spinal systems. Experimental data are lacking regarding the extent to which rehabilitative methods may influence these adaptations. An understanding of the plasticity of the muscular, skeletal, and spinal systems after paralysis may be important as new rehabilitative technologies emerge in the 21st century. Moreover, individuals injured today may become poor candidates for future scientific advancements (cure) if their neuromusculoskeletal systems are irreversibly impaired. The primary purpose of this paper is to explore the physiological properties of skeletal muscle as a result of spinal cord injury; secondarily, to consider associated changes at the skeletal and spinal levels. Muscular adaptations include a transformation to faster myosin, increased contractile speeds, shift to the right on the torque-frequency curve, increased fatigue, and enhanced doublet potentiation. These muscular adaptations may be prevented in individuals with acute paralysis and partially reversed in individuals with chronic paralysis. Moreover, the muscular changes may be coordinated with motor unit and spinal circuitry adaptations. Concurrently, skeletal adaptations, as measured by bone mineral density, show extensive loss within the first six months after paralysis. The underlying science governing neuromusculoskeletal adaptations after paralysis will help guide professionals as new rehabilitation strategies evolve in the future.
Figures


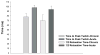


References
-
- Biering-Sorensen F, Bohr H, Schaadt O. Bone mineral content of the lumbar spine and lower extremities years after spinal cord lesion. Paraplegia. 1988;26:293–301. - PubMed
-
- Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–335. - PubMed
-
- Binder-Macleod ST, Barker CB. Use of a catchlike property of human skeletal muscle to reduce fatigue. Muscle Nerve. 1991;14:850–857. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

