Expression of functional interleukin-3 receptors on Hodgkin and Reed-Sternberg cells
- PMID: 11839579
- PMCID: PMC1850655
- DOI: 10.1016/S0002-9440(10)64878-X
Expression of functional interleukin-3 receptors on Hodgkin and Reed-Sternberg cells
Abstract
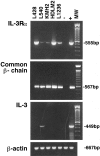
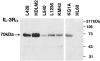
The human interleukin-3 receptor (IL-3R) is a heterodimeric complex consisting of an IL-3-specific alpha chain (IL-3Ralpha) and a common beta chain (beta(c)), this latter shared with the receptors for granulocyte-macrophage colony-stimulating factor and IL-5. Despite extensive research on cytokine circuitries regulating proliferation and survival of tumor cells in Hodgkin's disease (HD) the functional expression of IL-3Rs in this pathobiological entity has not yet been investigated. In the present study, we demonstrate that the great majority (>90%) of malignant Hodgkin and Reed-Sternberg cells of classic HD (19 of 19 analyzed cases) express IL-3Ralpha by immunostaining of frozen sections and cell suspensions from involved lymph nodes. Accordingly, HD cell lines (L428, KMH2, HDLM2, L1236) expressed the alpha and beta chains of IL-3R both at the mRNA and protein level, with a molecular size of IL-3Ralpha identical (70 kd) to that expressed by human myeloid cells. Exogenous IL-3 promoted the growth of cultured Hodgkin and Reed-Sternberg cells, such effect being potentiated by IL-9 co-stimulation, and was able to partially rescue tumor cells from apoptosis induced by serum deprivation. This data suggests an involvement of IL-3/IL-3R interactions in the cellular growth of HD through paracrine mechanisms.
Figures








Comment in
-
Interleukin-3 receptors in Hodgkin's disease.Am J Pathol. 2003 Jan;162(1):355-6; author reply 356-7. doi: 10.1016/s0002-9440(10)63827-8. Am J Pathol. 2003. PMID: 12507919 Free PMC article. No abstract available.
References
-
- Anastasi J, Bitter MA, Vardiman JW: The histopathologic diagnosis and subclassification of Hodgkin’s disease. Hematol Oncol Clin North Am 1989, 3:187-204 - PubMed
-
- Harris NL: Hodgkin’s disease: classification and differential diagnosis. Mod Pathol 1999, 12:159-175 - PubMed
-
- Gruss HJ, Kadin ME: Pathophysiology of Hodgkin’s disease: functional and molecular aspects. Baillieres Clin Hematol 1996, 9:417-446 - PubMed
-
- Gruss HJ, Pinto A, Duyster J, Poppema S, Herrmann F: Hodgkin’s disease: a tumor with disturbed immunological pathways. Immunol Today 1997, 18:156-163 - PubMed
-
- Pinto A, Gattei V, Zagonel V, Aldinucci D, Degan M, De Juiliis A, Rossi FM, Tassan Mazzocco F, Godeas C, Rupolo M, Poletto D, Gloghini A, Carbone A, Gruss HJ: Hodgkin’s disease: a disorder of dysregulated cellular cross-talk. Biotherapy 1998, 10:309-320 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

