Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells
- PMID: 11839588
- PMCID: PMC1850663
- DOI: 10.1016/S0002-9440(10)64887-0
Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells
Abstract
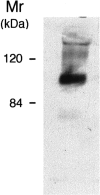
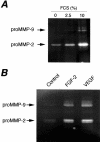
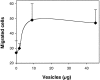
Production of matrix-degrading proteases, particularly matrix metalloproteinases (MMPs), by endothelial cells is a critical event during angiogenesis, the process of vessel neoformation that occurs in normal and pathological conditions. MMPs are known to be highly regulated at the level of synthesis and activation, however, little is known about the regulation of MMP secretion by endothelial cells. We found that cultured human umbilical vein endothelial cells shed vesicles (300 to 600 nm) originating from localized areas of the cell plasma membrane, as revealed by ultrastructural analysis. Normal and reverse zymography, Western blot, and immunogold analyses of the vesicles showed two gelatinases, MMP-2 and MMP-9, in both the active and proenzyme forms, the MT1-MMP proenzyme located on the external side of the vesicle membrane and the two inhibitors TIMP-1 and TIMP-2. Serum and the angiogenic factors, fibroblast growth factor-2 and vascular endothelial growth factor, stimulated the shedding of MMPs as vesicle components. Shedding the vesicle was rapid, as it was already completed after 4 hours. Addition of shed vesicles to human umbilical vein endothelial cells resulted in autocrine stimulation of invasion through a layer of reconstituted basement membrane (Matrigel) and cord formation on Matrigel. We conclude that endothelial cells shed MMP-containing vesicles and this may be a mechanism for regulating focalized proteolytic activity vital to invasive and morphogenic events during angiogenesis.
Figures

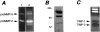

References
-
- Taylor DD, Black PH: Shedding of plasma membrane fragments. Steinberg M eds. Developmental Biology. 1986, :pp 33-57 Plenum Press, New York - PubMed
-
- Black PH: Shedding from the cell surface of normal and cancer cells. Advances in Cancer Research. 1980, :pp 75-197 New York, Academic Press - PubMed
-
- Dolo V, Ginestra A, Ghersi G, Nagase H, Vittorelli ML: Human breast carcinoma cells cultured in the presence of serum shed membrane vesicles rich in gelatinolytic activities. J Submicrosc Cytol Pathol 1994, 26:173-180 - PubMed
-
- Ginestra A, Monea S, Seghezzi G, Dolo V, Nagase H, Mignatti P, Vittorelli ML: Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT-1080 fibrosarcoma cells. J Biol Chem 1997, 272:17216-17221 - PubMed
-
- Dolo V, D’Ascenzo S, Violini S, Pompucci L, Festuccia C, Ginestra A, Vittorelli ML, Canevari S, Pavan A: Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin Exp Metastasis 1999, 7:131-140 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

