Quantitative trait loci influence renal disease progression in a mouse model of Alport syndrome
- PMID: 11839593
- PMCID: PMC1850644
- DOI: 10.1016/S0002-9440(10)64892-4
Quantitative trait loci influence renal disease progression in a mouse model of Alport syndrome
Abstract
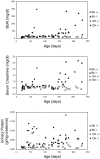
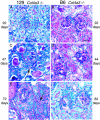
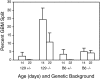
Alport syndrome is a human hereditary glomerulonephritis which results in end-stage renal failure (ESRF) in most cases. It is caused by mutations in any one of the collagen alpha3(IV), alpha4(IV), or alpha5(IV) chain genes (COL4A3-COL4A5). Patients carrying identical mutations can exhibit very different disease courses, suggesting that other genes or the environment influence disease progression. We previously generated a knockout mouse model of Alport syndrome by mutating Col4a3. Here, we show that genetic background strongly influences the timing of onset of disease and rate of progression to ESRF in these mice. On the 129X1/SvJ background, Col4a3 -/- mice reached ESRF at approximately 66 days of age, while on the C57BL/6J background, the mean age at ESRF was 194 days of age. This suggests the existence of modifier genes that influence disease progression. A detailed histopathological analysis revealed that glomerular basement membrane lesions typical of Alport syndrome were significantly more frequent in homozygotes on the 129X1/SvJ background than on the C57BL/6J background as early as two weeks of age, suggesting that modifier genes act by influencing glomerular basement membrane structure. Additional data indicated that differential physiological responses to basement membrane splitting also underlie the differences in disease progression. We attempted to map the modifier genes as quantitative trait loci (QTLs) using age at ESRF as the quantitative trait. Genome scans were performed on mice at the two extremes in a cohort of mutant F1 x C57BL/6J backcross mice. Analysis with Map Manager QT revealed QTLs linked to markers on chromosomes 9 and 16. A more detailed understanding of how these QTLs act could lead to new approaches for therapy in diverse renal diseases.
Figures




References
-
- Atkin CL, Gregory MC, Border WA: Alport syndrome. Schrier RW Gottschalk CW eds. Diseases of the Kidney. 1988, :pp 617-641 Little, Brown and Company, Boston
-
- Gubler M-C, Antignac C, Deschenes G, Knebelmann B, Hors-Cayla MC, Grunfeld J-P, Broyer M, Habib R: Genetic, clinical, and morphologic heterogeneity in Alport’s syndrome. Adv Nephrol 1993, 22:15-35 - PubMed
-
- Kashtan CE, Michael AF: Alport syndrome: from bedside to genome to bedside. Am J Kidney Dis 1993, 22:627-640 - PubMed
-
- Hudson BG, Reeders ST, Tryggvason K: Type IV collagen: structure, gene organization, and role in human diseases. J Biol Chem 1993, 268:26033-26036 - PubMed
-
- Antignac C: Molecular genetics of basement membranes: the paradigm of Alport syndrome. Kidney Int 1995, 47(Suppl 49):S29-S33 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

