Galectin-8 expression decreases in cancer compared with normal and dysplastic human colon tissue and acts significantly on human colon cancer cell migration as a suppressor
- PMID: 11839721
- PMCID: PMC1773143
- DOI: 10.1136/gut.50.3.392
Galectin-8 expression decreases in cancer compared with normal and dysplastic human colon tissue and acts significantly on human colon cancer cell migration as a suppressor
Abstract
Background and aims: Galectins are beta-galactoside binding proteins. This ability may have a bearing on cell adhesion and migration/proliferation in human colon cancer cells. In addition to galectins-1 and -3 studied to date, other members of this family not investigated in detail may contribute to modulation of tumour cell features. This evident gap has prompted us to extend galectin analysis beyond the two prototypes. The present study deals with the quantitative determination of immunohistochemical expression of galectin-8 in normal, benign, and malignant human colon tissue samples and in four human colon cancer models (HCT-15, LoVo, CoLo201, and DLD-1) maintained both in vitro as permanent cell lines and in vivo as nude mice xenografts. The role of galectin-8 (and its neutralising antibody) in cell migration was investigated in HCT-15, LoVo, CoLo201, and DLD-1 cell lines.
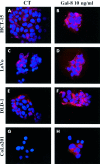
Methods: Immunohistochemical expression of galectin-8 and its overall ability to bind to sugar ligands (revealed glycohistochemically by means of biotinylated histochemically inert carrier bovine serum albumin with alpha- and beta-D-galactose, alpha-D-glucose, and lactose derivatives as ligands) were quantitatively determined using computer assisted microscopy. The presence of galectin-8 mRNA in the four human colon cancer cell lines was examined by reverse transcriptase-polymerase chain reaction. In vitro, cellular localisation of exogenously added galectin-8 in the culture media of these colon cancer cells was visualised by fluorescence microscopy. In vitro galectin-8 mediated effects (and the influence of its neutralising antibody) on migration levels of living HCT-15, LoVo, CoLo201, and DLD-1 cells were quantitatively determined by computer assisted phase contrast microscopy.
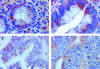
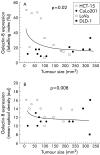
Results: A marked decrease in immunohistochemical expression of galectin-8 occurred with malignancy development in human colon tissue. Malignant colon tissue exhibited a significantly lower galectin-8 level than normal or benign tissue colon cancers; those with extensive invasion capacities (T3-4/N+/M+) harboured significantly less galectin-8 than colon cancers with localised invasion capacities (T1-2/N0/M0). The four experimental models (HCT-15, LoVo, CoLo201, and DLD-1) had more intense galectin-8 dependent staining in vitro than in vivo. Grafting the four experimental human colon cancer models onto nude mice enabled us to show that the immunohistochemical expression of galectin-8 was inversely related to tumour growth rate. In vitro, galectin-8 reduced the migration rate of only those human experimental models (HCT-15 and CoLo201) that exhibited the lowest growth rate in vivo.
Conclusions: Expression of galectin-8 correlated with malignancy development, with suppressor activity, as shown by analysis of clinical samples and xenografts. In vitro, only the two models with low growth rates were sensitive to the inhibitory potential of this galectin. Future investigations in this field should involve fingerprinting of these newly detected galectins, transcending the common focus on galectins-1 and -3.
Figures






References
-
- Gabius HJ. Endogenous lectins in tumors and the immune system. Cancer Invest 1987;5:39–46. - PubMed
-
- Raz A, Lotan R. Endogenous galactoside-binding lectins: a new class of functional tumor cell surface molecules related to metastasis. Cancer Metastasis Rev 1987;6:433–52. - PubMed
-
- Kim YJ, Varki A. Perspective on the significance of altered glycosylation of glycoproteins in cancer. Glycoconjugate J 1997;14:569–76. - PubMed
-
- Hakomori SI. Cancer-associated glycosphingolipid antigens: their structure, organisation, and function. Acta Anat 1998;161:79–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
