Different modes of hypertrophy in skeletal muscle fibers
- PMID: 11839766
- PMCID: PMC2174086
- DOI: 10.1083/jcb.200105147
Different modes of hypertrophy in skeletal muscle fibers
Abstract
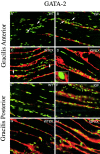
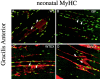
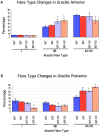
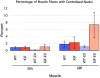
Skeletal muscles display a remarkable diversity in their arrangement of fibers into fascicles and in their patterns of innervation, depending on functional requirements and species differences. Most human muscle fascicles, despite their great length, consist of fibers that extend continuously from one tendon to the other with a single nerve endplate band. Other mammalian muscles have multiple endplate bands and fibers that do not insert into both tendons but terminate intrafascicularly. We investigated whether these alternate structural features may dictate different modes of cell hypertrophy in two mouse gracilis muscles, in response to expression of a muscle-specific insulin-like growth factor (IGF)-1 transgene (mIGF-1) or to chronic exercise. Both hypertrophic stimuli independently activated GATA-2 expression and increased muscle cross-sectional area in both muscle types, with additive effects in exercising myosin light chain/mIGF transgenic mice, but without increasing fiber number. In singly innervated gracilis posterior muscle, hypertrophy was characterized by a greater average diameter of individual fibers, and centralized nuclei. In contrast, hypertrophic gracilis anterior muscle, which is multiply innervated, contained longer muscle fibers, with no increase in average diameter, or in centralized nuclei. Different modes of muscle hypertrophy in domestic and laboratory animals have important implications for building appropriate models of human neuromuscular disease.
Figures







References
-
- Adams, D., and B. MacKay. 1961. The distribution of motor end-plates in mammalian muscles. Bibl. Anat. 2:153–154.
-
- Allen, D.L., B.C. Harrison, A. Maass, M.L. Bell, W.C. Byrnes, and L.A. Leinwand. 2001. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J. Appl. Physiol. 90:1900–1908. - PubMed
-
- Brown, I.E., T. Satoda, F.J. Richmond, and G.E. Loeb. 1998. Feline caudofemoralis muscle. Muscle fibre properties, architecture, and motor innervation. Exp. Brain Res. 121:76–91. - PubMed
-
- Clark, G. 1981. Methods for connective tissue. Staining Procedures. G. Clark, editor. Williams & Wilkins, Baltimore. 512.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

