Mouse Pop1 is required for muscle regeneration in adult skeletal muscle
- PMID: 11839816
- PMCID: PMC134701
- DOI: 10.1128/MCB.22.5.1504-1512.2002
Mouse Pop1 is required for muscle regeneration in adult skeletal muscle
Abstract
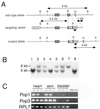
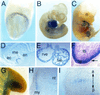
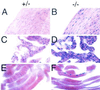
Popeye (Pop) genes are a novel gene family encoding putative transmembrane proteins predominantly present in striated and smooth muscle cells. In this study, a null mutation of Pop1 was generated by replacing the first coding exon of the Pop1 gene with the lacZ reporter gene. Homozygous mice lacking Pop1 were fertile and had a normal life span without any apparent phenotype. LacZ staining of tissues of heterozygous and homozygous Pop1-LacZ mice revealed strong expression in embryonic and fetal hearts. Pop1-LacZ was also expressed in the myotome and in myogenic progenitor cells within the limb and in smooth muscle cells of various organs. In the heart, Pop1-LacZ activity was downregulated postnatally in heterozygous mice but not in homozygous mice. Administration of the beta-adrenergic agonist isoproterenol led to a rapid increase in Pop1-LacZ activity in heterozygotes without induction at the transcriptional level, suggesting stabilization of the protein. No difference, however, was observed between homozygous and heterozygous mice in the ability to develop cardiac hypertrophy in response to isoproterenol. The capacity to regenerate skeletal muscle was tested after cardiotoxin injection into the hind limbs of hetero- and homozygous mice. In activated satellite cells of both genotypes, rapid activation of Pop1-LacZ expression was observed. In heterozygous animals, LacZ activity was only transiently elevated in muscle precursor cells undergoing fusion and in newly formed myotubes. In homozygotes, persistence of LacZ expression and a retarded ability to regenerate skeletal muscle were apparent, suggesting that Pop1 plays a role in muscle regeneration.
Figures


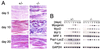

References
-
- Allen, R., C. Temm-Grove, S. Sheehan, and G. Rice. 1997. Methods in muscle biology. Methods Cell Biol. 52:155-176. - PubMed
-
- Andrée, B., T. Hillemann, G. Kessler-Icekson, T. Schmitt-John, H. Jockusch, H. H. Arnold, and T. Brand. 2000. Isolation and characterization of the novel popeye gene family expressed in skeletal muscle and heart. Dev. Biol. 223:371-382. - PubMed
-
- Brand, T., H. S. Sharma, and W. Schaper. 1993. Expression of nuclear proto-oncogenes in isoproterenol-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 25:1325-1337. - PubMed
-
- d'Albis, A., R. Couteaux, C. Janmot, and J. C. Mira. 1989. Myosin isoform transitions in regeneration of fast and slow muscles during postnatal development of the rat. Dev. Biol. 135:320-325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
