Interactions involving the Rad51 paralogs Rad51C and XRCC3 in human cells
- PMID: 11842112
- PMCID: PMC100332
- DOI: 10.1093/nar/30.4.1001
Interactions involving the Rad51 paralogs Rad51C and XRCC3 in human cells
Abstract
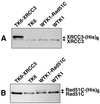
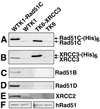
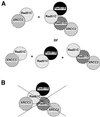
Homologous recombinational repair of DNA double-strand breaks and crosslinks in human cells is likely to require Rad51 and the five Rad51 paralogs (XRCC2, XRCC3, Rad51B/Rad51L1, Rad51C/Rad51L2 and Rad51D/Rad51L3), as has been shown in chicken and rodent cells. Previously, we reported on the interactions among these proteins using baculovirus and two- and three-hybrid yeast systems. To test for interactions involving XRCC3 and Rad51C, stable human cell lines have been isolated that express (His)6-tagged versions of XRCC3 or Rad51C. Ni2+-binding experiments demonstrate that XRCC3 and Rad51C interact in human cells. In addition, we find that Rad51C, but not XRCC3, interacts directly or indirectly with Rad51B, Rad51D and XRCC2. These results argue that there are at least two complexes of Rad51 paralogs in human cells (Rad51C-XRCC3 and Rad51B-Rad51C-Rad51D-XRCC2), both containing Rad51C. Moreover, Rad51 is not found in these complexes. X-ray treatment did not alter either the level of any Rad51 paralog or the observed interactions between paralogs. However, the endogenous level of Rad51C is moderately elevated in the XRCC3-overexpressing cell line, suggesting that dimerization between these proteins might help stabilize Rad51C.
Figures



References
-
- Bianco P.R., Tracy,R.B. and Kowalczykowski,S.C. (1998) DNA strand exchange proteins: a biochemical and physical comparison. Front. Biosci., 3, D570–D603. - PubMed
-
- Liu N., Lamerdin,J.E., Tebbs,R.S., Schild,D., Tucker,J.D., Shen,M.R., Brookman,K.W., Siciliano,M.J., Walter,C.A., Fan,W., Narayana,L.S., Zhou,Z.Q., Adamson,A.W., Sorensen,K.J., Chen,D.J., Jones,N.J. and Thompson,L.H. (1998) XRCC2 and XRCC3, new human Rad51-family members, promote chromosome stability and protect against DNA cross-links and other damages. Mol. Cell., 1, 783–793. - PubMed
-
- Johnson R.D., Liu,N. and Jasin,M. (1999) Mammalian XRCC2 promotes the repair of DNA double-strand breaks by homologous recombination. Nature, 401, 397–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

