Soybean ENOD40 encodes two peptides that bind to sucrose synthase
- PMID: 11842184
- PMCID: PMC122294
- DOI: 10.1073/pnas.022664799
Soybean ENOD40 encodes two peptides that bind to sucrose synthase
Abstract
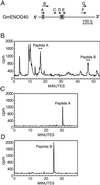
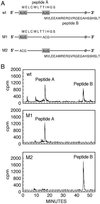
ENOD40 is expressed at an early stage in root nodule organogenesis in legumes. Identification of ENOD40 homologs in nonleguminous plants suggests that this gene may have a more general biological function. In vitro translation of soybean ENOD40 mRNA in wheat germ extracts revealed that the conserved nucleotide sequence at the 5' end (region I) encodes two peptides of 12 and 24 aa residues (peptides A and B). These peptides are synthesized de novo from very short, overlapping ORFs. Appropriate ORFs are present in all legume ENOD40s studied thus far. In this case small peptides are directly translated from polycistronic eukaryotic mRNA. The 24-aa peptide B was detected in nodules by Western blotting. Both peptides specifically bind to the same 93-kDa protein, which was affinity purified from soybean nodules. Using peptide mass fingerprinting, we identified this binding protein as nodulin 100, which is a subunit of sucrose synthase. Based on our data we suggest that ENOD40 peptides are involved in the control of sucrose use in nitrogen-fixing nodules.
Figures




References
-
- Minami E, Kouchi H, Cohn J R, Ogawa T, Stacey G. Plant J. 1996;10:23–32. - PubMed
-
- Kouchi H, Hata S. Mol Gen Genet. 1993;238:106–119. - PubMed
-
- Yang W C, Katinakis P, Hendriks P, Smolders A, de Vries F, Spee J, van Kammen A, Bisseling T, Franssen H. Plant J. 1993;3:573–585. - PubMed
-
- Kouchi H, Takane K, So R B, Ladha J K, Reddy P M. Plant J. 1999;18:121–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

