Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system
- PMID: 11842212
- PMCID: PMC122364
- DOI: 10.1073/pnas.042688199
Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system
Abstract
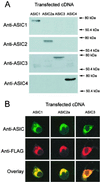
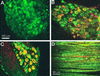
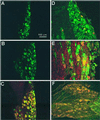
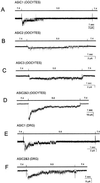
Acid-sensitive ion channels (ASIC) are proton-gated ion channels expressed in neurons of the mammalian central and peripheral nervous systems. The functional role of these channels is still uncertain, but they have been proposed to constitute mechanoreceptors and/or nociceptors. We have raised specific antibodies for ASIC1, ASIC2, ASIC3, and ASIC4 to examine the distribution of these proteins in neurons from dorsal root ganglia (DRG) and to determine their subcellular localization. Western blot analysis demonstrates that all four ASIC proteins are expressed in DRG and sciatic nerve. Immunohistochemical experiments and functional measurements of unitary currents from the ASICs with the patch-clamp technique indicate that ASIC1 localizes to the plasma membrane of small-, medium-, and large-diameter cells, whereas ASIC2 and ASIC3 are preferentially in medium to large cells. Neurons coexpressing ASIC2 and ASIC3 form predominantly heteromeric ASIC2-3 channels. Two spliced forms, ASIC2a and ASIC2b, colocalize in the same population of DRG neurons. Within cells, the ASICs are present mainly on the plasma membrane of the soma and cellular processes. Functional studies indicate that the pH sensitivity for inactivation of ASIC1 is much higher than the one for activation; hence, increases in proton concentration will inactivate the channel. These functional properties and localization in DRG have profound implications for the putative functional roles of ASICs in the nervous system.
Figures



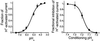
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

