Motility initiation in herring sperm is regulated by reverse sodium-calcium exchange
- PMID: 11842223
- PMCID: PMC122313
- DOI: 10.1073/pnas.042700899
Motility initiation in herring sperm is regulated by reverse sodium-calcium exchange
Abstract
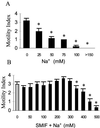
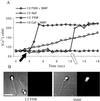
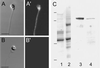
Sperm of the Pacific herring, Clupea pallasi, are unique in that they are immotile upon spawning in the environment. Herring sperm have evolved to remain motionless for up to several days after spawning, yet are still capable of fertilizing eggs. An egg chorion ligand termed "sperm motility initiation factor" (SMIF) induces motility in herring sperm and is required for fertilization. In this study, we show that SMIF induces calcium influx, sodium efflux, and a membrane depolarization in herring sperm. Sperm motility initiation by SMIF depended on decreased extracellular sodium (<350 mM) and could be induced in the absence of SMIF in very low sodium seawater. Motility initiation depended on > or =1 mM extracellular calcium. Calcium influx caused by SMIF involved both the opening of voltage-gated calcium channels and reverse sodium-calcium (Na(+)/Ca(2+)) exchange. Membrane depolarization was slightly inhibited by a calcium channel blocker and markedly inhibited by a Na(+)/Ca(2+) exchange inhibitor. Sodium efflux caused by SMIF-initiated motility was observed when using both extracellular and intracellular sodium probes. A Na(+)/Ca(2+) exchange antigen was shown to be present on the surface of the sperm, primarily over the midpiece, by using an antibody to the canine Na(+)/Ca(2+) exchanger. This antibody recognized a 120-kDa protein that comigrated with the canine myocyte Na(+)/Ca(2+) exchanger. Sperm of Pacific herring are now shown to use reverse Na(+)/Ca(2+) exchange in motility initiation. This mechanism of regulation of motility initiation may have evolved for both maintenance of immotility after spawning as well as ligand-induced motility initiation.
Figures





Similar articles
-
Two egg-derived molecules in sperm motility initiation and fertilization in the Pacific herring (Clupea pallasi).Int J Dev Biol. 2008;52(5-6):743-52. doi: 10.1387/ijdb.072566gc. Int J Dev Biol. 2008. PMID: 18649286
-
Role of the Na+/Ca2+ exchanger in calcium homeostasis and human sperm motility regulation.Cell Motil Cytoskeleton. 2006 Feb;63(2):66-76. doi: 10.1002/cm.20108. Cell Motil Cytoskeleton. 2006. PMID: 16374831
-
Sperm motility initiation factor is a minor component of the Pacific herring egg chorion.Dev Growth Differ. 1996 Apr;38(2):193-202. doi: 10.1046/j.1440-169X.1996.t01-1-00009.x. Dev Growth Differ. 1996. PMID: 37282292
-
Structure and Function of Ion Channels Regulating Sperm Motility-An Overview.Int J Mol Sci. 2021 Mar 23;22(6):3259. doi: 10.3390/ijms22063259. Int J Mol Sci. 2021. PMID: 33806823 Free PMC article. Review.
-
Sperm motility in fishes. (II) Effects of ions and osmolality: a review.Cell Biol Int. 2006 Jan;30(1):1-14. doi: 10.1016/j.cellbi.2005.06.004. Epub 2005 Nov 8. Cell Biol Int. 2006. PMID: 16278089 Review.
Cited by
-
The role of alkalinization-induced Ca2+ influx in sperm motility activation of a viviparous fish Redtail Splitfin (Xenotoca eiseni).Biol Reprod. 2018 Dec 1;99(6):1159-1170. doi: 10.1093/biolre/ioy150. Biol Reprod. 2018. PMID: 29982498 Free PMC article.
-
Chemical and physical guidance of fish spermatozoa into the egg through the micropyle†,‡.Biol Reprod. 2017 Apr 1;96(4):780-799. doi: 10.1093/biolre/iox015. Biol Reprod. 2017. PMID: 28371886 Free PMC article.
-
Sperm proteins in teleostean and chondrostean (sturgeon) fishes.Fish Physiol Biochem. 2009 Nov;35(4):567-81. doi: 10.1007/s10695-008-9261-y. Epub 2008 Sep 23. Fish Physiol Biochem. 2009. PMID: 18810648 Review.
-
Regulation of sperm motility in Eastern oyster (Crassostrea virginica) spawning naturally in seawater with low salinity.PLoS One. 2021 Mar 18;16(3):e0243569. doi: 10.1371/journal.pone.0243569. eCollection 2021. PLoS One. 2021. PMID: 33735238 Free PMC article.
-
Activation of motility and chemotaxis in the spermatozoa: From invertebrates to humans.Reprod Med Biol. 2005 May 3;4(2):101-114. doi: 10.1111/j.1447-0578.2005.00099.x. eCollection 2005 Jun. Reprod Med Biol. 2005. PMID: 29699215 Free PMC article. Review.
References
-
- Morisawa M. Zool Sci. 1994;11:647–662. - PubMed
-
- Gatti J-L, Billard R, Christen R. J Cell Physiol. 1990;143:546–554. - PubMed
-
- Boitano S, Omoto C K. J Cell Sci. 1991;98:343–349. - PubMed
-
- Tanimoto S, Kudo Y, Nakazawa T, Morisawa M. Mol Reprod Dev. 1994;39:409–414. - PubMed
-
- Darszon A, Labarca P, Nishigaki T, Espinosa F. Physiol Rev. 1999;79:481–510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

