Latent transforming growth factor beta-binding protein-3 and fibulin-1C interact with the extracellular domain of the heparin-binding EGF-like growth factor precursor
- PMID: 11846885
- PMCID: PMC65547
- DOI: 10.1186/1471-2121-3-2
Latent transforming growth factor beta-binding protein-3 and fibulin-1C interact with the extracellular domain of the heparin-binding EGF-like growth factor precursor
Abstract
Background: The membrane-bound cell-surface precursor and soluble forms of heparin-binding epidermal growth factor-like growth factor (HB-EGF) contribute to many cellular developmental processes. The widespread occurrence of HB-EGF in cell and tissue types has led to observations of its role in such cellular and tissue events as tumor formation, cell migration, extracellular matrix formation, wound healing, and cell adherence. Several studies have reported the involvement of such extracellular matrix proteins as latent transforming growth factor beta-binding protein, TGF-beta, and fibulin-1 in some of these processes. To determine whether HB-EGF interacts with extracellular matrix proteins we used the extracellular domain of proHB-EGF in a yeast two-hybrid system to screen a monkey kidney cDNA library. cDNA clones containing nucleotide sequences encoding domains of two proteins were obtained and their derived amino acid sequences were evaluated.
Results: From approximately equal to 3 x 10(6) screened monkey cDNA clones, cDNA clones were recovered that contained nucleotide sequences encoding domains of the monkey latent transforming growth factor-beta binding protein-3 (MkLTBP-3) and fibulin-1C protein. The amino acid sequence derived from the MkLTBP-3 gene shared 98.6% identity with human LTBP-3 and 86.7% identity with mouse LTBP-3 amino acid sequences. The amino acid sequence derived from the monkey fibulin-1C gene shared 97.2% identity with human fibulin-1C. Yeast two-hybrid screens indicate that LTBP-3 and fibulin-1C interact with proHB-EGF through their calcium-binding EGF-like modules.
Conclusions: The interactions of the extracellular domain of proHB-EGF with LTBP-3 and fibulin-1C suggest novel functions for HB-EGF between cell and tissue surfaces.
Figures
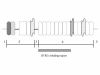
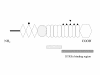
Similar articles
-
Identification and characterization of LTBP-2, a novel latent transforming growth factor-beta-binding protein.J Biol Chem. 1994 Dec 23;269(51):32469-78. J Biol Chem. 1994. PMID: 7798248
-
Importance of the major extracellular domain of CD9 and the epidermal growth factor (EGF)-like domain of heparin-binding EGF-like growth factor for up-regulation of binding and activity.J Biol Chem. 2000 Jun 16;275(24):18284-90. doi: 10.1074/jbc.M907971199. J Biol Chem. 2000. PMID: 10749879
-
Latent transforming growth factor-beta binding proteins (LTBPs)--structural extracellular matrix proteins for targeting TGF-beta action.Cytokine Growth Factor Rev. 1999 Jun;10(2):99-117. doi: 10.1016/s1359-6101(99)00010-6. Cytokine Growth Factor Rev. 1999. PMID: 10743502 Review.
-
BAG-1 is a novel cytoplasmic binding partner of the membrane form of heparin-binding EGF-like growth factor: a unique role for proHB-EGF in cell survival regulation.J Biol Chem. 2001 Aug 10;276(32):30127-32. doi: 10.1074/jbc.M010237200. Epub 2001 May 4. J Biol Chem. 2001. PMID: 11340068
-
Heparin-binding EGF-like growth factor: a juxtacrine growth factor.Cytokine Growth Factor Rev. 2000 Dec;11(4):335-44. doi: 10.1016/s1359-6101(00)00013-7. Cytokine Growth Factor Rev. 2000. PMID: 10959080 Review.
Cited by
-
Fibulin-1c regulates transforming growth factor-β activation in pulmonary tissue fibrosis.JCI Insight. 2019 Jul 25;5(16):e124529. doi: 10.1172/jci.insight.124529. JCI Insight. 2019. PMID: 31343988 Free PMC article.
-
CCN3 and calcium signaling.Cell Commun Signal. 2003 Aug 15;1(1):1. doi: 10.1186/1478-811X-1-1. Cell Commun Signal. 2003. PMID: 14606958 Free PMC article.
-
APP Binds to the EGFR Ligands HB-EGF and EGF, Acting Synergistically with EGF to Promote ERK Signaling and Neuritogenesis.Mol Neurobiol. 2021 Feb;58(2):668-688. doi: 10.1007/s12035-020-02139-2. Epub 2020 Oct 2. Mol Neurobiol. 2021. PMID: 33009641
-
LTBPs, more than just an escort service.J Cell Biochem. 2012 Feb;113(2):410-8. doi: 10.1002/jcb.23385. J Cell Biochem. 2012. PMID: 22223425 Free PMC article. Review.
-
Cell Derived Matrix Fibulin-1 Associates With Epidermal Growth Factor Receptor to Inhibit Its Activation, Localization and Function in Lung Cancer Calu-1 Cells.Front Cell Dev Biol. 2020 Jul 3;8:522. doi: 10.3389/fcell.2020.00522. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32719793 Free PMC article.
References
-
- Davis-Fleischer KM, Besner GE. Structure and function of heparin-binding EGF-like growth factor (HB-EGF). Frontiers in Bioscience. 1998;3:d288–299. - PubMed
-
- Naglich JG, Metherall JE, Russell DW, Eidels L. Expression cloning of diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992;69:1051–1061. - PubMed
-
- Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. - PubMed
-
- Higashiyama S, Lau K, Besner GE, Abraham JA, Klagsbrun M. Structure of heparin-binding EGF-like growth factor. Multiple forms, primary structure, and glycosylation of the mature protein. J Biol Chem. 1992;267:6205–6212. - PubMed
-
- Goishi K, Higashiyama S, Klagsbrun M, Nakano N, Umata T, Ishikawa M, Mekada E, Taniguchi N. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtracrine to paracrine growth factor activity. Mo Biol Cell. 1995;6:967–980. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

