Regulation of receptor protein-tyrosine phosphatase alpha by oxidative stress
- PMID: 11847098
- PMCID: PMC125870
- DOI: 10.1093/emboj/21.4.493
Regulation of receptor protein-tyrosine phosphatase alpha by oxidative stress
Abstract
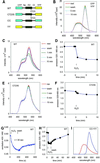
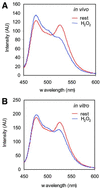
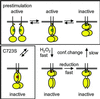
The presence of two protein-tyrosine phosphatase (PTP) domains is a striking feature in most transmembrane receptor PTPs (RPTPs). The function of the generally inactive membrane-distal PTP domain (RPTP-D2) is unknown. Here we report that an intramolecular interaction between the spacer region (Sp) and the C-terminus in RPTPalpha prohibited intermolecular interactions. Interestingly, stress factors such as H(2)O(2), UV and heat shock induced reversible, free radical-dependent, intermolecular interactions between RPTPalpha and RPTPalpha-SpD2, suggesting an inducible switch in conformation and binding. The catalytic site cysteine of RPTPalpha-SpD2, Cys723, was required for the H(2)O(2) effect on RPTPalpha. H(2)O(2) induced a rapid, reversible, Cys723-dependent conformational change in vivo, as detected by fluorescence resonance energy transfer, with cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) flanking RPTPalpha-SpD2 in a single chimeric protein. Importantly, H(2)O(2) treatment stabilized RPTPalpha dimers, resulting in inactivation. We propose a model in which oxidative stress induces a conformational change in RPTPalpha-D2, leading to stabilization of RPTPalpha dimers, and thus to inhibition of RPTPalpha activity.
Figures





References
-
- Aricescu A.R., Fulga,T.A., Cismasiu,V., Goody,R.S. and Szedlacsek,S.E. (2001) Intramolecular interactions in protein tyrosine phosphatase RPTPµ: kinetic evidence. Biochem. Biophys. Res. Commun., 280, 319–327. - PubMed
-
- Bae Y.S., Kang,S.W., Seo,M.S., Baines,I.C., Tekle,E., Chock,P.B. and Rhee,S.G. (1997) Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem., 272, 217–221. - PubMed
-
- Bastiaens P.I. and Pepperkok,R. (2000) Observing proteins in their natural habitat: the living cell. Trends Biochem. Sci., 25, 631–637. - PubMed
-
- Bilwes A.M., den Hertog,J., Hunter,T. and Noel,J.P. (1996) Structural basis for inhibition of receptor protein-tyrosine phosphatase-α by dimerization. Nature, 382, 555–559. - PubMed
-
- Blanchetot C. and den Hertog,J. (2000) Multiple interactions between receptor protein-tyrosine phosphatase (RPTP) α and membrane-distal protein-tyrosine phosphatase domains of various RPTPs. J. Biol. Chem., 275, 12446–12452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

