The PreS2 activator MHBs(t) of hepatitis B virus activates c-raf-1/Erk2 signaling in transgenic mice
- PMID: 11847101
- PMCID: PMC125852
- DOI: 10.1093/emboj/21.4.525
The PreS2 activator MHBs(t) of hepatitis B virus activates c-raf-1/Erk2 signaling in transgenic mice
Abstract
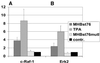
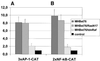
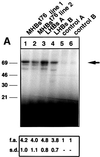
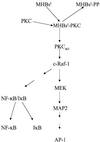
The large hepatitis B virus (HBV) surface protein (LHBs) and C-terminally truncated middle size surface proteins (MHBs(t)) form the family of the PreS2 activator proteins of HBV. Their transcriptional activator function is based on the cytoplasmic orientation of the PreS2 domain. MHBs(t) activators are paradigmatic for this class of activators. Here we report that MHBs(t) is protein kinase C (PKC)-dependently phosphorylated at Ser28. The integrity of the phosphorylation site is essential for the activator function. MHBs(t) triggers PKC-dependent activation of c-Raf-1/Erk2 signaling that is a prerequisite for MHBs(t)-dependent activation of AP-1 and NF-kappaB. To analyze the pathophysiological relevance of these data in vivo, transgenic mice were established that produce the PreS2 activator MHBs(t) specifically in the liver. In these mice, a permanent PreS2-dependent specific activation of c-Raf-1/Erk2 signaling was observed, resulting in an increased hepatocyte proliferation rate. In transgenics older than 15 months, an increased incidence of liver tumors occurs. These data suggest that PreS2 activators LHBs and MHBs(t) exert a tumor promoter-like function by activation of key enzymes of proliferation control.
Figures













Similar articles
-
The PreS2 activators of the hepatitis B virus: activators of tumour promoter pathways.Recent Results Cancer Res. 1998;154:315-29. doi: 10.1007/978-3-642-46870-4_23. Recent Results Cancer Res. 1998. PMID: 10027012 Review.
-
The hepatitis B virus large surface protein (LHBs) is a transcriptional activator.Virology. 1996 Nov 1;225(1):235-9. doi: 10.1006/viro.1996.0594. Virology. 1996. PMID: 8918553
-
The hepatitis B virus protein MHBs(t) sensitizes hepatoma cells to TRAIL-induced apoptosis through ERK2.Apoptosis. 2007 Oct;12(10):1827-36. doi: 10.1007/s10495-007-0114-4. Apoptosis. 2007. PMID: 17701086
-
Integrity of c-Raf-1/MEK signal transduction cascade is essential for hepatitis B virus gene expression.Oncogene. 2003 May 1;22(17):2604-10. doi: 10.1038/sj.onc.1206320. Oncogene. 2003. PMID: 12730674
-
Quantification of large and middle proteins of hepatitis B virus surface antigen (HBsAg) as a novel tool for the identification of inactive HBV carriers.Gut. 2018 Nov;67(11):2045-2053. doi: 10.1136/gutjnl-2017-313811. Epub 2017 Sep 26. Gut. 2018. PMID: 28951526
Cited by
-
Phosphoproteomics Unravel HBV Triggered Rewiring of Host Phosphosignaling Events.Int J Mol Sci. 2022 May 4;23(9):5127. doi: 10.3390/ijms23095127. Int J Mol Sci. 2022. PMID: 35563518 Free PMC article.
-
Hepatocellular carcinoma and hepatitis B surface protein.World J Gastroenterol. 2016 Feb 14;22(6):1943-52. doi: 10.3748/wjg.v22.i6.1943. World J Gastroenterol. 2016. PMID: 26877602 Free PMC article. Review.
-
Hepatitis B Virus Middle Protein Enhances IL-6 Production via p38 MAPK/NF-κB Pathways in an ER Stress-Dependent Manner.PLoS One. 2016 Jul 19;11(7):e0159089. doi: 10.1371/journal.pone.0159089. eCollection 2016. PLoS One. 2016. PMID: 27434097 Free PMC article.
-
CRISPR/Cas9-mediated knockout of HBsAg inhibits proliferation and tumorigenicity of HBV-positive hepatocellular carcinoma cells.J Cell Biochem. 2018 Nov;119(10):8419-8431. doi: 10.1002/jcb.27050. Epub 2018 Jun 15. J Cell Biochem. 2018. PMID: 29904948 Free PMC article.
-
Transactivation of proto-oncogene c-Myc by hepatitis B virus transactivator MHBst167.Oncol Lett. 2014 Aug;8(2):803-808. doi: 10.3892/ol.2014.2190. Epub 2014 May 28. Oncol Lett. 2014. PMID: 25009657 Free PMC article.
References
-
- Beasley R.P., Lin,C.C., Hwang,L.Y. and Chien,C.S. (1981) Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22,707 men in Taiwan. Lancet, 2, 1129–1133. - PubMed
-
- Caroll M. and May,W.S. (1994) Protein kinase C-mediated serine phosphorylation directly activates raf-1 in murine hematopoietic cells. J. Biol. Chem., 269, 1249–1256. - PubMed
-
- Chisari F.V., Pinkert,C.A., Milich,D.R., Filippi,P., McLachlan,A., Palmiter,R.D. and Brinster,R.L. (1985) A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science, 230, 1157–1160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

