Conserved properties of hydrogenosomal and mitochondrial ADP/ATP carriers: a common origin for both organelles
- PMID: 11847105
- PMCID: PMC125860
- DOI: 10.1093/emboj/21.4.572
Conserved properties of hydrogenosomal and mitochondrial ADP/ATP carriers: a common origin for both organelles
Abstract
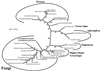
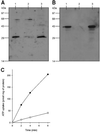
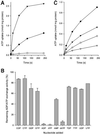
Mitochondria are one of the hallmarks of eukaryotic cells, exporting ATP in exchange for cytosolic ADP using ADP/ATP carriers (AAC) located in the inner mitochondrial membrane. In contrast, several evolutionarily important anaerobic eukaryotes lack mitochondria but contain hydrogenosomes, peculiar organelles of controversial ancestry that also supply ATP but, like some fermentative bacteria, make molecular hydrogen in the process. We have now identified genes from two species of the hydrogenosome-containing fungus Neocallimastix that have three-fold sequence repeats and signature motifs that, along with phylogenetic analysis, identify them as AACs. When expressed in a mitochondrial AAC- deficient yeast strain, the hydrogenosomal protein was correctly targeted to the yeast mitochondria inner membrane and yielded mitochondria able to perform ADP/ATP exchange. Characteristic inhibitors of mitochondrial AACs blocked adenine nucleotide exchange by the Neocallimastix protein. Thus, our data demonstrate that fungal hydrogenosomes and yeast mitochondria use the same pathway for ADP/ATP exchange. These experiments provide some of the strongest evidence yet that yeast mitochondria and Neocallimastix hydrogenosomes are but two manifestations of the same fundamental organelle.
Figures


Similar articles
-
Multiple origins of hydrogenosomes: functional and phylogenetic evidence from the ADP/ATP carrier of the anaerobic chytrid Neocallimastix sp.Mol Microbiol. 2002 Jun;44(6):1441-54. doi: 10.1046/j.1365-2958.2002.02959.x. Mol Microbiol. 2002. PMID: 12067335
-
A divergent ADP/ATP carrier in the hydrogenosomes of Trichomonas gallinae argues for an independent origin of these organelles.Mol Microbiol. 2004 Mar;51(5):1439-46. doi: 10.1111/j.1365-2958.2004.03918.x. Mol Microbiol. 2004. PMID: 14982636
-
Presence of a member of the mitochondrial carrier family in hydrogenosomes: conservation of membrane-targeting pathways between hydrogenosomes and mitochondria.Mol Cell Biol. 2000 Apr;20(7):2488-97. doi: 10.1128/MCB.20.7.2488-2497.2000. Mol Cell Biol. 2000. PMID: 10713172 Free PMC article.
-
The ADP and ATP transport in mitochondria and its carrier.Biochim Biophys Acta. 2008 Oct;1778(10):1978-2021. doi: 10.1016/j.bbamem.2008.04.011. Epub 2008 May 2. Biochim Biophys Acta. 2008. PMID: 18510943 Review.
-
Chemical, immunological, enzymatic, and genetic approaches to studying the arrangement of the peptide chain of the ADP/ATP carrier in the mitochondrial membrane.J Bioenerg Biomembr. 1993 Oct;25(5):459-72. doi: 10.1007/BF01108403. J Bioenerg Biomembr. 1993. PMID: 8132486 Review.
Cited by
-
Degenerate mitochondria.EMBO Rep. 2005 Jun;6(6):525-30. doi: 10.1038/sj.embor.7400440. EMBO Rep. 2005. PMID: 15940286 Free PMC article. Review.
-
Heterologous transporters from anaerobic fungi bolster fluoride tolerance in Saccharomyces cerevisiae.Metab Eng Commun. 2019 Apr 11;9:e00091. doi: 10.1016/j.mec.2019.e00091. eCollection 2019 Dec. Metab Eng Commun. 2019. PMID: 31016136 Free PMC article.
-
Isolation and Biochemical Characterization of Six Anaerobic Fungal Strains from Zoo Animal Feces.Microorganisms. 2021 Aug 3;9(8):1655. doi: 10.3390/microorganisms9081655. Microorganisms. 2021. PMID: 34442734 Free PMC article.
-
Triplicate genes for mitochondrial ADP/ATP carriers in the aerobic yeast Yarrowia lipolytica are regulated differentially in the absence of oxygen.Mol Genet Genomics. 2005 Mar;273(1):84-91. doi: 10.1007/s00438-005-1107-z. Epub 2005 Feb 2. Mol Genet Genomics. 2005. PMID: 15688220
-
Organelles in Blastocystis that blur the distinction between mitochondria and hydrogenosomes.Curr Biol. 2008 Apr 22;18(8):580-5. doi: 10.1016/j.cub.2008.03.037. Epub 2008 Apr 10. Curr Biol. 2008. PMID: 18403202 Free PMC article.
References
-
- Akhmanova A., Voncken,F., van Alen,A.T., van Hoek,H.A., Boxma,B., Vogels,G., Veenhuis,M. and Hackstein,J.H. (1998) A hydrogenosome with a genome. Nature, 396, 527–528. - PubMed
-
- Altschul S.F., Gish,W., Miller,W., Myers,E.W. and Lipman,D.J. (1990) Basic local alignment search tool. J. Mol. Biol., 215, 403–410. - PubMed
-
- Benchimol M., Durand,R. and Almeida,J.C.A. (1997) A double membrane surrounds the hydrogenosomes of the anaerobic fungus Neocallimastix frontalis. FEMS Microbiol. Lett., 154, 277–282. - PubMed
-
- Biagini G.A., van der Giezen,M., Hill,B., Winters,C. and Lloyd,D. (1997) Ca2+ accumulation in the hydrogenosomes of Neocallimastix frontalis L2: a mitochondrial-like physiological role. FEMS Microbiol. Lett., 149, 227–232.
-
- Bjerrum O.J. and Heegaard,N.H.H. (1988) CRC Handbook of Immunoblotting of Proteins. CRC Press, Boca Raton, FL.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

