The role of aromatic residues in the hydrophobic core of the villin headpiece subdomain
- PMID: 11847290
- PMCID: PMC2373478
- DOI: 10.1110/ps.22202
The role of aromatic residues in the hydrophobic core of the villin headpiece subdomain
Abstract
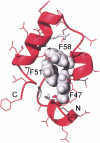
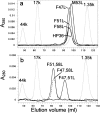
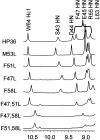
Small autonomously folding proteins are of interest as model systems to study protein folding, as the same molecule can be used for both experimental and computational approaches. The question remains as to how well these minimized peptide model systems represent larger native proteins. For example, is the core of a minimized protein tolerant to mutation like larger proteins are? Also, do minimized proteins use special strategies for specifying and stabilizing their folded structure? Here we examine these questions in the 35-residue autonomously folding villin headpiece subdomain (VHP subdomain). Specifically, we focus on a cluster of three conserved phenylalanine (F) residues F47, F51, and F58, that form most of the hydrophobic core. These three residues are oriented such that they may provide stabilizing aromatic-aromatic interactions that could be critical for specifying the fold. Circular dichroism and 1D-NMR spectroscopy show that point mutations that individually replace any of these three residues with leucine were destabilized, but retained the native VHP subdomain fold. In pair-wise replacements, the double mutant that retains F58 can adopt the native fold, while the two double mutants that lack F58 cannot. The folding of the double mutant that retains F58 demonstrates that aromatic-aromatic interactions within the aromatic cluster are not essential for specifying the VHP subdomain fold. The ability of the VHP subdomain to tolerate mutations within its hydrophobic core indicates that the information specifying the three dimensional structure is distributed throughout the sequence, as observed in larger proteins. Thus, the VHP subdomain is a legitimate model for larger, native proteins.
Figures


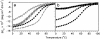
References
-
- Burley, S. and Petsko, G. 1986. Dimerization energetics of benzene and aromatic amino acid side chains. J. Am. Chem. Soc. 108 7995–8001.
-
- Burley, S.K. and Petsko, G.A. 1988. Weakly polar interactions in proteins. Adv. Protein Chem. 39 125–189. - PubMed
-
- Cantor, C.R. and Schimmel, P.R. 1980. Biophysical chemistry, vol. 3. W.H. Freeman and Co., New York.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

