The pharmacokinetic properties of intramuscular artesunate and rectal dihydroartemisinin in uncomplicated falciparum malaria
- PMID: 11849191
- PMCID: PMC1874553
- DOI: 10.1046/j.0306-5251.2001.01519.x
The pharmacokinetic properties of intramuscular artesunate and rectal dihydroartemisinin in uncomplicated falciparum malaria
Abstract
Aims: To obtain pharmacokinetic data for artesunate (ARTS) and its active metabolite dihydroartemisinin (DHA) following i.m. ARTS and rectal DHA administration.
Methods: Twelve Vietnamese patients with uncomplicated falciparum malaria were randomized to receive either i.v. or i.m. ARTS (120 mg), with the alternative preparation given 8 h later in an open crossover design. A further 12 patients were given i.v. ARTS (120 mg) at 0 h and rectal DHA (160 mg) 8 h later.
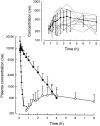
Results: Following i.v. bolus, ARTS had a peak concentration of 42 microm (16 mg l(-1), elimination t1/2 = 3.2 min, CL = 2.8 l h(-1) kg(-1) and V = 0.22 l kg(-1) . The Cmax for DHA was 9.7 microm (2.7 mg l(-1) ), t1/2 = 59 min, CL = 0.64 l h(-1) kg(-1) and V = 0.8 l kg(-1) . Following i.m. ARTS, Cmax was 2.3 microm (3.7 mg l(-1)), the apparent t1/2 = 41 min, CL = 2.9 l h(-1) kg(-1) and V = 2.6 l kg(-1). The relative bioavailability of DHA was 88%, Cmax was 4.1 microm (1.16 mg l(-1)) and t1/2 = 64 min. In the rectal DHA study, relative bioavailability of DHA was 16%.
Conclusions: For patients with uncomplicated falciparum malaria i.m. ARTS is a suitable alternative to i.v. ARTS, at equal doses. To achieve plasma DHA concentrations equivalent to parenteral administration of ARTS, rectal DHA should be given at approximately four-fold higher milligram doses. Further studies are needed to determine whether these recommendations can be applied to patients with severe malaria.
Figures


References
-
- van Agtmael MA, Eggelte TA, van Boxtel CJ. Artemisinin drugs in the treatment of malaria: from medicinal herb to registered medication. Trends Pharmacol Sci. 1999;20:199–205. - PubMed
-
- Navaratnam V, Mansor SM, Sit N, Grace JM, Li Q, Olliaro P. Pharmacokinetics of artemisinin-type compounds. Clin Pharmacokinet. 2000;39:255–270. - PubMed
-
- de Vries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52:818–836. - PubMed
-
- Barradell LB, Fitton A. Artesunate. A review of its pharmacology and therapeutic efficacy in the treatment of malaria. Drugs. 1995;50:714–741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

