IgG4 breaking the rules
- PMID: 11849310
- PMCID: PMC1782638
- DOI: 10.1046/j.0019-2805.2001.01341.x
IgG4 breaking the rules
Abstract
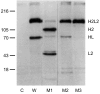
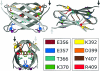
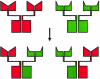
Immunoglobulin G4 (IgG4) antibodies have been known for some time to be functionally monovalent. Recently, the structural basis for this monovalency has been elucidated: the in vivo exchange of IgG half-molecules (one H-plus one L-chain) among IgG4. This process results in bispecific antibodies that in most situations will behave as functionally monovalent antibodies. The structural basis for the abnormal behaviour of IgG4 seems to be largely the result of a single amino acid change relative to human IgG1: the change of a proline in core hinge of IgG1 to serine. This results in a marked shift in the equilibrium between interchain disulphide bridges and intrachain disulphide bridges, which for IgG4 results in 25-75% absence of a covalent interaction between the H-chains. Because of strong non-covalent interactions between the CH3 domains (and possibly also between the CH1 domain and the trans-CH2 domain) IgG4 is a stable four-chain molecule and does not easily exchange half-molecules under standard physiological conditions in vitro. We postulate that the exchange is catalysed in vivo by protein disulphide isomerase (PDI) and/or FcRn (the major histocompatibility complex (MHC)-related Fc receptor) during transit of IgG4 in the endosomal pathway in endothelial cells. Because IgG4 is predominantly expressed under conditions of chronic antigen exposure, the biological relevance of this exchange of half-molecules is that it generates antibodies that are unable to form large immune complexes and therefore have a low potential for inducing immune inflammation. In contrast to monovalent immunoglobulin fragments, these scrambled immunoglobulins have a normal half-life. The significance of the ensuing bispecificity needs further evaluation, because this will be relevant only in situations where high IgG4 responses are found to two unrelated antigens that happen to be present in the body at the same time and place. In this context the significance of IgG4 autoreactivity might have to be re-evaluated. The main function of IgG4, however, is presumably to interfere with immune inflammation induced by complement-fixing antibodies, or, in the case of helminth infection or allergy, by IgE antibodies.
Figures



References
-
- Angal S, King DJ, Bodmer MW, Turner A, Lawson ADG, Roberts G, Pedley B, Adair JR. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol Immunol. 1993;30:105–8. - PubMed
-
- Schuurman J, Perdok GJ, Gorter AD, Aalberse RC. The inter-heavy chain disulfide bonds of IgG4 are in equilibrium with intra-chain disulfide bonds. Mol Immunol. 2001;38:1–8. - PubMed
-
- Aalberse RC, Van Der Gaag R, Van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130:722–6. - PubMed
-
- Van Der Zee JS, Van Swieten P, Aalberse RC. Serologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J Immunol. 1986;137:3566–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

