Prostaglandin D(2), its metabolite 15-d-PGJ(2), and peroxisome proliferator activated receptor-gamma agonists induce apoptosis in transformed, but not normal, human T lineage cells
- PMID: 11849312
- PMCID: PMC1782633
- DOI: 10.1046/j.0019-2805.2001.01340.x
Prostaglandin D(2), its metabolite 15-d-PGJ(2), and peroxisome proliferator activated receptor-gamma agonists induce apoptosis in transformed, but not normal, human T lineage cells
Abstract
Prostaglandin D(2) (PGD(2)) is abundantly produced by mast cells, platelets, and alveolar macrophages and has been proposed as a key immunoregulatory lipid mediator. 15-Deoxy-Delta(12,14)-PGJ(2) (15-d-PGJ(2)), a key PGD(2) metabolite, is under intense study as a potential anti-inflammatory mediator. Little is known about PGD(2) or the role of 15-d-PGJ(2), if any, in regulating the activities of human T lineage cells. In this report we demonstrate that both PGD(2) and 15-d-PGJ(2) have potent antiproliferative effects, and in fact kill human T lymphocyte lines derived from malignant cells by an apoptotic mechanism. Interestingly, normal human T cells were not similarly affected. Although the T lymphocyte lines express mRNA for the PGD(2) receptor (DP-R), a potent DP receptor agonist, BW245C, did not inhibit the proliferation or viability of the cells, suggesting an alternative mechanism of action. PGD(2) and 15-d-PGJ(2) can bind to the peroxisome proliferator activated receptor-gamma (PPAR-gamma) which is implicated in lipid metabolism and apoptosis. Exposure to synthetic PPAR-gamma ligands (e.g. ciglitazone, troglitazone) mimicked the inhibitory responses of PGD(2) and 15-d-PGJ(2), and induced apoptosis in the transformed T cells consistent with a PPAR-gamma-dependent mechanism. These observations suggest that PPAR-gamma ligands (which may include PGD2) provide strong apoptotic signals to transformed, but not normal T lymphocytes. Thus, the efficacy of utilizing PPAR-gamma and its ligands as therapeutics for human T cell cancers needs to be further evaluated.
Figures



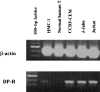

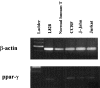



Comment in
-
Prostaglandin and PPAR control of immune cell function.Immunology. 2002 Jan;105(1):20-2. doi: 10.1046/j.0019-2805.2001.01361.x. Immunology. 2002. PMID: 11849311 Free PMC article. No abstract available.
References
-
- Ito S, Narumiya S, Hayaishi O. Prostaglandin D2: a biochemical perspective. Prostaglandins Leukot Essent Fatty Acids. 1989;37:219–34. - PubMed
-
- Urade Y, Hayaishi O. Prostaglandin D synthase: structure and function. Vitam Horm. 2000;58:89–120. - PubMed
-
- Boie Y, Sawyer N, Slipetz DM, Metters KM, Abramovitz M. Molecular cloning and characterization of the human prostanoid DP receptor. J Biol Chem. 1995;270:18910–6. - PubMed
-
- Clay CE, Namen AM, Atsumi G, et al. Influence of J series prostaglandins on apoptosis and tumorigenesis of breast cancer cells. Carcinogenesis. 1999;20:1905–11. - PubMed
-
- Colville-Nash PR, Qureshi SS, Willis D, Willoughby DA. Inhibition of inducible nitric oxide synthase by peroxisome proliferator-activated receptor agonists: correlation with induction of heme oxygenase 1. J Immunol. 1998;161:978–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

