Influence of CD4 T cells and the source of major histocompatibility complex class II-restricted peptides on cytotoxic T-cell priming by dendritic cells
- PMID: 11849314
- PMCID: PMC1782635
- DOI: 10.1046/j.0019-2805.2001.01343.x
Influence of CD4 T cells and the source of major histocompatibility complex class II-restricted peptides on cytotoxic T-cell priming by dendritic cells
Erratum in
- Immunology 2002 May;106(1):122-3
Abstract
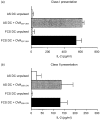
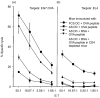
We have previously reported that bone marrow derived dendritic cells (DC) pulsed with major histocompatibility complex (MHC) class I-restricted peptide efficiently prime a cytotoxic T lymphocyte (CTL) response in vivo. Here we assess the involvement of CD4(+) T cells in the induction of CD8(+) CTL by DC by testing the ability of class II-deficient (C2D) DC, class II mutant (Alpha beta mut) DC and autologous serum generated DC (AS DC) to present class I-restricted antigens in vitro and in vivo. DC generated from the bone marrow of class II knockout mice and transgenic mice expressing a mutant class II that can not bind CD4 were phenotypically similar to wild type (wt) DC, except with regard to MHC class II expression. The C2D and Alpha beta mut DC, though fully capable of presenting the class I-restricted ovalbumin (OVA) peptide to a T-cell hybridoma in vitro, failed to prime a CTL response in vivo. Restoration of class II expression on C2D DC allowed priming of a CTL response; thus, the defect in CTL priming was indeed caused by the absence of class II expression. Likewise, DC generated in autologous serum were unable to prime a CTL response as these DC only express 'self' class II epitopes and therefore would not activate syngeneic CD4(+) T cells. Addition of exogenous class II epitopes rescued the ability of AS DC to prime a CTL response. These observations provide convincing evidence that efficient CTL induction by DC in vivo requires concomitant presentation of class II epitopes for CD4(+) T-cell induction.
Figures





Similar articles
-
NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo.J Immunol. 2003 Mar 1;170(5):2540-8. doi: 10.4049/jimmunol.170.5.2540. J Immunol. 2003. PMID: 12594280
-
Bone marrow-generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes.J Exp Med. 1995 Jul 1;182(1):255-60. doi: 10.1084/jem.182.1.255. J Exp Med. 1995. PMID: 7540653 Free PMC article.
-
Active CD4+ helper T cells directly stimulate CD8+ cytotoxic T lymphocyte responses in wild-type and MHC II gene knockout C57BL/6 mice and transgenic RIP-mOVA mice expressing islet beta-cell ovalbumin antigen leading to diabetes.Autoimmunity. 2008 Nov;41(7):501-11. doi: 10.1080/08916930802069256. Autoimmunity. 2008. PMID: 18855194
-
Dendritic cells as stimulator cells of MHC class I-restricted immune responses.Adv Exp Med Biol. 1995;378:341-5. doi: 10.1007/978-1-4615-1971-3_76. Adv Exp Med Biol. 1995. PMID: 8526088 Review.
-
[Towards novel tuberculosis and leprosy vaccine development: the role of Th1-inducing peptide in cytotoxic T cell differentiation].Nihon Hansenbyo Gakkai Zasshi. 2013 Dec;82(3):111-7. doi: 10.5025/hansen.82.111. Nihon Hansenbyo Gakkai Zasshi. 2013. PMID: 24579458 Review. Japanese.
Cited by
-
Paradigm Shift in Dendritic Cell-Based Immunotherapy: From in vitro Generated Monocyte-Derived DCs to Naturally Circulating DC Subsets.Front Immunol. 2014 Apr 11;5:165. doi: 10.3389/fimmu.2014.00165. eCollection 2014. Front Immunol. 2014. PMID: 24782868 Free PMC article. Review.
-
The history, evolution, and clinical use of dendritic cell-based immunization strategies in the therapy of brain tumors.J Neurooncol. 2003 Aug-Sep;64(1-2):161-76. doi: 10.1007/BF02700031. J Neurooncol. 2003. PMID: 12952297 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

