Inhibition of B16 melanoma experimental metastasis by interferon-gamma through direct inhibition of cell proliferation and activation of antitumour host mechanisms
- PMID: 11849319
- PMCID: PMC1782640
- DOI: 10.1046/j.0019-2805.2001.01342.x
Inhibition of B16 melanoma experimental metastasis by interferon-gamma through direct inhibition of cell proliferation and activation of antitumour host mechanisms
Abstract
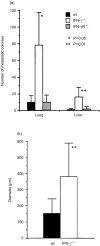
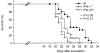
Interferon-gamma (IFN-gamma) has pleiotropic activities other than its antivirus action, including cell growth inhibition, natural killer (NK) cell and cytotoxic T lymphocyte (CTL) activation, and angiogenesis inhibitory activity, and these activities are supposed to be involved in its antitumour activity. However, it has not been completely elucidated which activity is mainly involved in the tumour suppression in vivo. In this study, we analysed inhibitory mechanisms of endogenous IFN-gamma against B16 melanoma experimental metastasis. After intravenous injection of tumour cells, tumour deposits in the lungs and liver were increased and life span was shorter in IFN-gamma(-/-) mice, indicating important roles for IFN-gamma in antitumour mechanisms. Interestingly, tumour deposits were not increased in IFN-gamma receptor (R)(-/-) mice. Furthermore, only low levels of cell-mediated immunity against the tumour and activation of NK cells were observed, indicating that antimetastatic effects of IFN-gamma is not mediated by host cells. The survival period of B16 melanoma-bearing IFN-gamma R(-/-) mice was, however, shorter than wild-type mice. These observations suggest that IFN-gamma prevents B16 melanoma experimental metastasis by directly inhibiting the cell growth, although antitumour host functions may also be involved in a later phase.
Figures







Similar articles
-
IFN-γ production by lung NK cells is critical for the natural resistance to pulmonary metastasis of B16 melanoma in mice.J Leukoc Biol. 2011 Oct;90(4):777-85. doi: 10.1189/jlb.0411208. Epub 2011 Jun 28. J Leukoc Biol. 2011. PMID: 21712396
-
Interleukin-12 activates NK cells for IFN-gamma-dependent and NKT cells for IFN-gamma-independent antimetastatic activity.Eur Cytokine Netw. 1999 Dec;10(4):541-8. Eur Cytokine Netw. 1999. PMID: 10586121
-
Interferon-gamma treatment of B16 melanoma cells: opposing effects for non-adaptive and adaptive immune defense and its reflection by metastatic spread.Int J Cancer. 1988 Feb 15;41(2):256-66. doi: 10.1002/ijc.2910410217. Int J Cancer. 1988. PMID: 3123403
-
Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity.Cold Spring Harb Perspect Biol. 2019 Mar 1;11(3):a028480. doi: 10.1101/cshperspect.a028480. Cold Spring Harb Perspect Biol. 2019. PMID: 29661791 Free PMC article. Review.
-
Interferon-gamma and resistance to bacterial infections.APMIS. 1993 Jan;101(1):1-17. doi: 10.1111/j.1699-0463.1993.tb00073.x. APMIS. 1993. PMID: 8457320 Review.
Cited by
-
Inhibition of breast cancer metastasis to the lungs with UBS109.Oncotarget. 2018 Nov 16;9(90):36102-36109. doi: 10.18632/oncotarget.26302. eCollection 2018 Nov 16. Oncotarget. 2018. PMID: 30546830 Free PMC article.
-
STAT3 Knockdown in B16 Melanoma by siRNA Lipopolyplexes Induces Bystander Immune Response In Vitro and In Vivo.Transl Oncol. 2011 Jun;4(3):178-88. doi: 10.1593/tlo.11100. Epub 2011 Jun 1. Transl Oncol. 2011. PMID: 21633673 Free PMC article.
-
CD160 Promotes NK Cell Functions by Upregulating Glucose Metabolism and Negatively Correlates With HIV Disease Progression.Front Immunol. 2022 Aug 19;13:854432. doi: 10.3389/fimmu.2022.854432. eCollection 2022. Front Immunol. 2022. PMID: 36110864 Free PMC article.
-
Interplay of microRNAs, transcription factors and target genes: linking dynamic expression changes to function.Nucleic Acids Res. 2013 Mar 1;41(5):2817-31. doi: 10.1093/nar/gks1471. Epub 2013 Jan 17. Nucleic Acids Res. 2013. PMID: 23335783 Free PMC article.
-
NFAT1 transcription factor is central in the regulation of tissue microenvironment for tumor metastasis.Cancer Immunol Immunother. 2011 Apr;60(4):537-46. doi: 10.1007/s00262-010-0964-4. Epub 2011 Jan 12. Cancer Immunol Immunother. 2011. PMID: 21225259 Free PMC article.
References
-
- Kaplan DH, Schreiber RH. The interferons: biochemistry and biology. In: Theze J, editor. The Cytokine Network and Immune Functions. New York: Oxford University Press; 1999. pp. 111–24.
-
- van den Broek MF, Muller U, Huang S, Zinkemagel RM, Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev. 1995;148:5–18. - PubMed
-
- Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–95. - PubMed
-
- Ossina NK, Cannas A, Powers VC, et al. Interferon-γ modulates a p53-independent apoptotic pathway and apoptosis-related gene expression. J Biol Chem. 1997;272:16351–7. - PubMed
-
- Jagus R, Joshi B, Barber GN. PKR, apoptosis and cancer. Int J Biochem Cell Biol. 1999;31:123–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

