Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors
- PMID: 11850253
- PMCID: PMC127499
- DOI: 10.1128/AAC.46.3.716-723.2002
Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors
Abstract
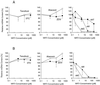
Drug-associated dysfunction of mitochondria is believed to play a role in the etiology of the various adverse symptoms that occur in human immunodeficiency virus (HIV)-infected patients treated with the nucleoside reverse transcriptase inhibitors (NRTIs). Tenofovir, a nucleotide analog recently approved for use in the treatment of HIV infection, was evaluated in vitro for its potential to cause mitochondrial toxicity and was compared to currently used NRTIs. Treatment with tenofovir (3 to 300 microM) for up to 3 weeks produced no significant changes in mitochondrial DNA (mtDNA) levels in human hepatoblastoma (HepG2) cells, skeletal muscle cells (SkMCs), or renal proximal tubule epithelial cells. The potencies of inhibition of mtDNA synthesis by the NRTIs tested were zalcitabine (ddC) > didanosine (ddI) > stavudine > zidovudine (ZDV) > lamivudine = abacavir = tenofovir, with comparable relative effects in the three cell types. Unlike ddC and ddI, tenofovir did not affect cellular expression of COX II and COX IV, two components of the mitochondrial cytochrome c oxidase complex. Lactate production was elevated by less than 20% in HepG2 cells or SkMCs following treatment with 300 microM tenofovir. In contrast, lactate synthesis increased by >200% in the presence of 300 microM ZDV. Thus, treatment of various human cell types with tenofovir at concentrations that greatly exceed those required for it both to have in vitro anti-HIV type 1 activity in peripheral blood mononuclear cells (50% effective concentration, 0.2 microM) and to achieve therapeutically relevant levels in plasma (maximum concentrations in plasma, 0.8 to 1.3 microM) is not associated with mitochondrial toxicity.
Figures
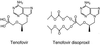
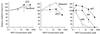

References
-
- Anderson, T. D., A. Davidovich, D. Feldman, T. J. Sprinkle, J. Arezzo, C. Brosnan, R. O. Calderon, L. H. Fossom, J. T. DeVries, and G. H. DeVries. 1994. Mitochondrial schwannopathy and peripheral myelinopathy in a rabbit model of dideoxycytidine neurotoxicity. Lab. Investig. 70:724-739. - PubMed
-
- Arnaudo, E., M. Dalakas, S. Shanske, C. T. Moraes, S. DiMauro, and E. A. Schon. 1991. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet 337:508-510. - PubMed
-
- Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. - PMC - PubMed
-
- Benbrik, E., P. Chariot, S. Bonavaud, M. Ammi-Said, E. Frisdal, C. Rey, R. Gherardi, and G. Barlovatz-Meimon. 1997. Cellular and mitochondrial toxicity of zidovudine (AZT), didanosine (ddI) and zalcitabine (ddC) on cultured human muscle cells. J. Neurol. Sci. 149:19-25. - PubMed
-
- Bissuel, F., F. Bruneel, F. Habersetzer, D. Chassard, L. Cotte, M. Chevallier, J. Bernuau, J. C. Lucet, and C. Trepo. 1994. Fulminant hepatitis with severe lactate acidosis in HIV-infected patients on didanosine therapy. J. Intern. Med. 235:367-371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

