Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth
- PMID: 11850458
- PMCID: PMC6757578
- DOI: 10.1523/JNEUROSCI.22-04-01303.2002
Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth
Abstract
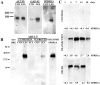
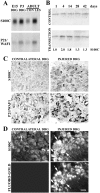
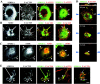
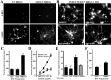
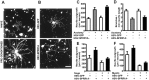
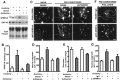
The ability of neurons to regenerate an axon after injury is determined by both the surrounding environment and factors intrinsic to the damaged neuron. We have used cDNA microarrays to survey those genes induced during successful sciatic nerve regeneration. The small proline-rich repeat protein 1A (SPRR1A) is not detectable in uninjured neurons but is induced by >60-fold after peripheral axonal damage. The protein is localized to injured neurons and axons. sprr1a is one of a group of epithelial differentiation genes, including s100c and p21/waf, that are coinduced in neurons by axotomy. Overexpressed SPRR1A colocalizes with F-actin in membrane ruffles and augments axonal outgrowth on a range of substrates. In axotomized sensory neurons, reduction of SPRR1A function restricts axonal outgrowth. Neuronal SPRR1A may be a significant contributor to successful nerve regeneration.
Figures

References
-
- Allen BG, Durussel I, Walsh MP, Cox JA. Characterization of the Ca2+-binding properties of calgizzarin (S100C) isolated from chicken gizzard smooth muscle. Biochem Cell Biol. 1996;74:687–694. - PubMed
-
- Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Skene JH. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci. 2001;4:38–43. - PubMed
-
- Chong MS, Woolf CJ, Haque NS, Anderson PN. Axonal regeneration from injured dorsal roots into the spinal cord of adult rats. J Comp Neurol. 1999;410:42–54. - PubMed
-
- Cox LS. Multiple pathways control cell growth and transformation: overlapping and independent activities of p53 and p21Cip1/WAF1/Sdi1. J Pathol. 1997;183:134–140. - PubMed
-
- Dickson BJ. Rho GTPases in growth cone guidance. Curr Opin Neurobiol. 2001;11:103–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
