Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure
- PMID: 11850459
- PMCID: PMC6757568
- DOI: 10.1523/JNEUROSCI.22-04-01316.2002
Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure
Abstract
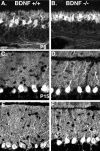
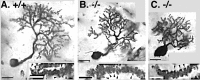
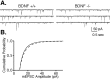
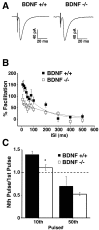
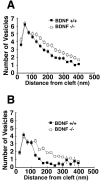
Neurotrophins are key regulators of neuronal survival and function. Here we show that TrkB, the receptor for brain-derived neurotrophic factor (BDNF), is located at parallel fiber to Purkinje cell (PF/PC) synapses of the cerebellum. To determine the effects of TrkB receptor activation on synapse formation and function, we examined the parallel fiber to Purkinje cell synapses of mice with a targeted deletion of the BDNF gene. Although Purkinje cell dendrites are abnormal in BDNF -/- mice, PF/PC synapses are still able to form. Immunohistochemical analysis of mutant animals revealed the formation of numerous PF/PC synapses with the appropriate apposition of presynaptic and postsynaptic proteins. These synapses are functional, and no differences were detected in the waveform of evoked EPSCs, the amplitude of spontaneous mini-EPSCs, or the response to prolonged 10 Hz stimulus trains. However, paired-pulse facilitation, a form of short-term plasticity, is significantly decreased in BDNF -/- mice. Detailed ultrastructural analysis of the presynaptic terminals demonstrated that this change in synaptic function is accompanied by an increase in the total number of synaptic vesicles in mutant mice and a decrease in the proportion of vesicles that are docked. These data suggest that BDNF regulates both the mechanisms that underlie short-term synaptic plasticity and the steady-state relationship between different vesicle pools within the terminal.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
