Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons
- PMID: 11850465
- PMCID: PMC6757552
- DOI: 10.1523/JNEUROSCI.22-04-01385.2002
Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons
Abstract
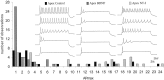
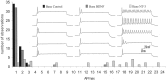
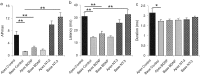
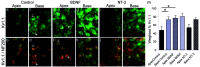
It is now well established that sensory neurons and receptors display characteristic morphological and electrophysiological properties tailored to their functions. This is especially evident in the auditory system, where cells are arranged tonotopically and are highly specialized for precise coding of frequency- and timing-dependent auditory information. Less well understood, however, are the mechanisms that give rise to these biophysical properties. We have provided insight into this issue by using whole-cell current-clamp recordings and immunocytochemistry to show that BDNF and NT-3, neurotrophins found normally in the cochlea, have profound effects on the firing properties and ion channel distribution of spiral ganglion neurons in the murine cochlea. Exposure of neurons to BDNF caused all neurons, regardless of their original cochlear position, to display characteristics of the basal neurons. Conversely, NT-3 caused cells to show the properties of apical neurons. These results are consistent with oppositely oriented gradients of these two neurotrophins and/or their high-affinity receptors along the tonotopic map, and they suggest that a combination of neurotrophins are necessary to establish the characteristic firing features of postnatal spiral ganglion neurons.
Figures




References
-
- Adamson CL. PhD thesis. The State University of New Jersey, Rutgers; 2001. Differential distribution of voltage-gated ion channels in spiral ganglion neurons.
-
- Adamson CL, Perney TM, Peng L, Davis RL. Immunohistochemical distribution of potassium channel subunits in murine spiral ganglion neurons. Soc Neurosci Abstr. 1999;25:667.10.
-
- Airaksinen MS, Koltzenburg M, Lewin GR, Masu Y, Helbig C, Wolf E, Brem G, Toyka KV, Thoenen H, Meyer M. Specific subtypes of cutaneous mechanoreceptors require neurotrophin-3 following peripheral target innervation. Neuron. 1996;16:287–295. - PubMed
-
- Anniko M, Arnold W, Stigbrand T, Strom A. The human spiral ganglion. ORL J Otorhinolaryngol Relat Spec. 1995;57:68–77. - PubMed
-
- Baldwin TJ, Tsaur ML, Lopez GA, Jan YN, Jan LY. Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel. Neuron. 1991;7:471–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
