Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats
- PMID: 11850474
- PMCID: PMC6757554
- DOI: 10.1523/JNEUROSCI.22-04-01480.2002
Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats
Abstract
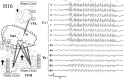
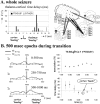
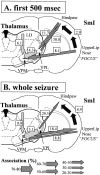
Absence seizures are the most pure form of generalized epilepsy. They are characterized in the electroencephalogram by widespread bilaterally synchronous spike-wave discharges (SWDs), which are the reflections of highly synchronized oscillations in thalamocortical networks. To reveal network mechanisms responsible for the initiation and generalization of the discharges, we studied the interrelationships between multisite cortical and thalamic field potentials recorded during spontaneous SWDs in the freely moving WAG/Rij rat, a genetic model of absence epilepsy. Nonlinear association analysis revealed a consistent cortical "focus" within the peri-oral region of the somatosensory cortex. The SWDs recorded at other cortical sites consistently lagged this focal site, with time delays that increased with electrode distance (corresponding to a mean propagation velocity of 1.4 m/sec). Intra-thalamic relationships were more complex and could not account for the observed cortical propagation pattern. Cortical and thalamic sites interacted bi-directionally, whereas the direction of this coupling could vary throughout one seizure. However, during the first 500 msec, the cortical focus was consistently found to lead the thalamus. These findings argue against the existence of one common subcortical pacemaker for the generation of generalized spike-wave discharges characteristic for absence seizures in the rat. Instead, the results suggest that a cortical focus is the dominant factor in initiating the paroxysmal oscillation within the corticothalamic loops, and that the large-scale synchronization is mediated by ways of an extremely fast intracortical spread of seizure activity. Analogous mechanisms may underlie the pathophysiology of human absence epilepsy.
Figures







References
-
- Avanzini G, Panzica F, De Curtis M. The role of the thalamus in vigilance, epileptogenic mechanisms. Clin Neurophysiol. 2000;111[Suppl 2]:S19–S26. - PubMed
-
- Avoli M, Gloor P, Kostopoulos G, Gotman J. An analysis of penicillin-induced generalized spike and wave discharges using simultaneous recordings of cortical and thalamic single neurons. J Neurophysiol. 1983;50:819–837. - PubMed
-
- Buzsáki G. The thalamic clock: emergent network properties. Neuroscience. 1991;41:351–364. - PubMed
-
- Chapin JK, Lin CS. Mapping the body representation in the SI cortex of anesthetized and awake rats. J Comp Neurol. 1984;229:199–213. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
