Effects of inhibitors of the lipo-oxygenase family of enzymes on the store-operated calcium current I(CRAC) in rat basophilic leukaemia cells
- PMID: 11850504
- PMCID: PMC2290129
- DOI: 10.1113/jphysiol.2001.012826
Effects of inhibitors of the lipo-oxygenase family of enzymes on the store-operated calcium current I(CRAC) in rat basophilic leukaemia cells
Abstract
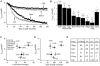
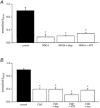
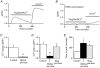
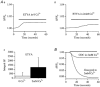
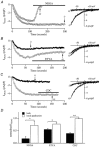
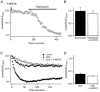
In non-excitable cells, the major Ca2+ entry pathway is the store-operated pathway in which emptying of intracellular Ca2+ stores activates Ca2+ channels in the plasma membrane. In many cell types, store-operated influx gives rise to a Ca2+-selective current called I(CRAC) (Ca2+ release-activated Ca2+ current). Using both the whole-cell patch clamp technique to measure I(CRAC) directly and fluorescent Ca2+ imaging, we have examined the role of the lipo-oxygenase pathway in the activation of store-operated Ca2+ entry in the RBL-1 rat basophilic leukaemia cell-line. Pretreatment with a variety of structurally distinct lipo-oxygenase inhibitors all reduced the extent of I(CRAC), whereas inhibition of the cyclo-oxygenase enzymes was without effect. The inhibition was still seen in the presence of the broad protein kinase blocker staurosporine, or when Na+ was used as the charge carrier through CRAC channels. The lipo-oxygenase blockers released Ca2+ from intracellular stores but this was not associated with subsequent Ca2+ entry. Lipo-oxygenase blockers also reduced both the amount of Ca2+ that could subsequently be released by the combination of thapsigargin and ionomycin in Ca2+-free solution and the Ca2+ influx component that occurred when external Ca2+ was re-admitted. The inhibitors were much less effective if applied after I(CRAC) had been activated. This inhibition of I(CRAC) could not be rescued by dialysis with 5(S)-hydroxyperoxyeicosa-6E,8Z,11Z,14Z,tetraenoic acid (5-HPETE), the first product of the 5-lipo-oxygenase pathway. Our findings indicate that exposure to pharmacological tools that inhibit the lipo-oxygenase enzymes all decrease the extent of activation of the current. Our results raise the possibility that a lipo-oxygenase might be involved in the activation of I(CRAC). Alternative explanations are also discussed.
Figures


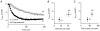

Similar articles
-
An examination of the secretion-like coupling model for the activation of the Ca2+ release-activated Ca2+ current I(CRAC) in RBL-1 cells.J Physiol. 2001 Apr 1;532(Pt 1):55-71. doi: 10.1111/j.1469-7793.2001.0055g.x. J Physiol. 2001. PMID: 11283225 Free PMC article.
-
Slow feedback inhibition of calcium release-activated calcium current by calcium entry.J Biol Chem. 1998 Jun 12;273(24):14925-32. doi: 10.1074/jbc.273.24.14925. J Biol Chem. 1998. PMID: 9614097
-
Ca2+-calmodulin-dependent facilitation and Ca2+ inactivation of Ca2+ release-activated Ca2+ channels.J Biol Chem. 2005 Mar 11;280(10):8776-83. doi: 10.1074/jbc.M409619200. Epub 2004 Dec 20. J Biol Chem. 2005. PMID: 15611075
-
Effects of phosphatidylinositol kinase inhibitors on the activation of the store-operated calcium current ICRAC in RBL-1 cells.Pflugers Arch. 2001 Jun;442(3):391-5. doi: 10.1007/s004240100546. Pflugers Arch. 2001. PMID: 11484770
-
Store-operated calcium channels.Physiol Rev. 2005 Apr;85(2):757-810. doi: 10.1152/physrev.00057.2003. Physiol Rev. 2005. PMID: 15788710 Review.
Cited by
-
Unveiling some FDA-approved drugs as inhibitors of the store-operated Ca2+ entry pathway.Sci Rep. 2017 Oct 16;7(1):12881. doi: 10.1038/s41598-017-13343-x. Sci Rep. 2017. PMID: 29038464 Free PMC article.
-
Store-operated Ca2+ entry: dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane.J Physiol. 2003 Mar 1;547(Pt 2):333-48. doi: 10.1113/jphysiol.2002.034140. Epub 2003 Feb 7. J Physiol. 2003. PMID: 12576497 Free PMC article.
-
The whole-cell Ca2+ release-activated Ca2+ current, ICRAC , is regulated by the mitochondrial Ca2+ uniporter channel and is independent of extracellular and cytosolic Na.J Physiol. 2020 May;598(9):1753-1773. doi: 10.1113/JP276551. Epub 2019 Feb 6. J Physiol. 2020. PMID: 30582626 Free PMC article.
-
Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels.J Biol Chem. 2011 Apr 29;286(17):14795-803. doi: 10.1074/jbc.M111.220582. Epub 2011 Feb 16. J Biol Chem. 2011. PMID: 21325277 Free PMC article.
-
Cysteinyl leukotriene type I receptor desensitization sustains Ca2+-dependent gene expression.Nature. 2012 Jan 9;482(7383):111-5. doi: 10.1038/nature10731. Nature. 2012. PMID: 22230957 Free PMC article.
References
-
- Barowski D, Parekh AB. Monovalent cation permeability and Ca2+ block of the store-operated Ca2+ current ICRAC in rat basophilic leukemia cells. Pflügers Archiv. 2002 (in the Press) - PubMed
-
- Beaven MA, Rogers J, Moore JP, Hesketh TR, Smith GA, Metcalfe JC. The mechanism of the calcium signal and correlation with histamine release in 2H3 cells. Journal of Biological Chemistry. 1984;259:7129–7136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

