Comparison of the properties of CLCA1 generated currents and I(Cl(Ca)) in murine portal vein smooth muscle cells
- PMID: 11850505
- PMCID: PMC2290135
- DOI: 10.1113/jphysiol.2001.013170
Comparison of the properties of CLCA1 generated currents and I(Cl(Ca)) in murine portal vein smooth muscle cells
Abstract
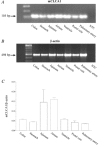
Calcium-activated chloride currents (I(Cl(Ca))) have been recorded in various smooth muscle cells but, to date, there has been no information as to the molecular nature of the channel underlying this conductance. We have characterised native I(Cl(Ca)) in freshly dispersed smooth muscle cells isolated from murine portal vein using whole-cell voltage clamp. I(Cl(Ca)) exhibited time-dependent activation at depolarised potentials and rapid deactivation upon repolarisation. The reversal potential of I(Cl(Ca)) was close to the theoretical equilibrium potential (E(Cl)) and was shifted by replacement of external Cl- by SCN- or isethionate. Dithiothreitol (DTT, 1 mM), a blocker of CLCA1, had no effect on the I(Cl(Ca)) current in myocytes. RT-PCR demonstrated the expression of mCLCA1 transcripts, but not mCLCA3 transcripts, in various murine smooth muscle cells including portal vein, as well as cardiomyocytes, and the levels of mCLCA1 transcriptional expression were quantified by real time quantitative RT-PCR. Stable transfection of HEK293 cells with the cDNA encoding mCLCA1 cloned from murine portal vein smooth muscle yielded a current with notable differences in Ca2+ sensitivity, channel kinetics and modulation by DTT from the native I(Cl(Ca)). However, there was some similarity in the pore properties and these data suggest that mCLCA1 alone does not comprise the Cl- channel in portal vein smooth muscle cells.
Figures


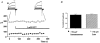

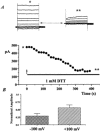
 ) and +100 mV (
) and +100 mV ( ) after 5 min application of 1 m
) after 5 min application of 1 mReferences
-
- Agnel M, Vermat T, Culouscou JM. Identification of three novel members of the calcium-dependent chloride channel (CaCC) family predominantly expressed in the digestive tract and trachea. FEBS Letters. 1999;455:295–301. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. - PubMed
-
- Arreola J, Melvin JE, Begenisich T. Differences in regulation of Ca2+-activated Cl− channels in colonic and parotid secretory cells. American Journal of Physiology. 1998;274:C161–166. - PubMed
-
- Cunningham SA, Awayda MS, Bubien JK, Ismailov II, Arrate MP, Berdiev BK, Benos DJ, Fuller CM. Cloning of an epithelial chloride channel from bovine trachea. Journal of Biological Chemistry. 1995;270:31016–31026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

