Rho and rho kinase modulation of barrier properties: cultured endothelial cells and intact microvessels of rats and mice
- PMID: 11850521
- PMCID: PMC2290121
- DOI: 10.1113/jphysiol.2001.013117
Rho and rho kinase modulation of barrier properties: cultured endothelial cells and intact microvessels of rats and mice
Abstract
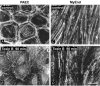
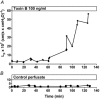
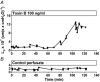
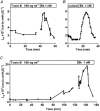
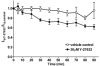
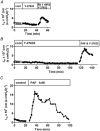
Previous experiments using cultured endothelial monolayers indicate that Rho-family small GTPases are involved in modulation of endothelial monolayer permeability by regulating assembly of the cellular actin filament scaffold, activity of myosin-based contractility and junctional distribution of the Ca2+-dependent endothelial cell adhesion molecule, VE-cadherin. We investigated these mechanisms using both cultured endothelial cells (from porcine pulmonary artery and mouse heart) and vascular endothelium in situ (mouse aorta, and individually perfused venular microvessels of mouse and rat mesentery). Exposure to Clostridium difficile toxin B (100 ng x ml(-1)) inactivated 50-90% of all endothelial Rho proteins within 60-90 min. This was accompanied by considerable reduction of actin filament stress fibres and junctional F-actin in cultured endothelial monolayers and in mouse aortic endothelium in situ. Also, VE-cadherin became discontinuous along endothelial junctions. Inhibition of Rho kinase with Y-27632 (30 microM) for 90-120 min induced F-actin reduction both in vitro and in situ but did not cause redistribution or reduction of VE-cadherin staining. Perfusion of microvessels with toxin B increased basal hydraulic permeability (L(p)) but did not attenuate the transient increase in L(p) of microvessels exposed to bradykinin. Perfusion of microvessels with Y-27632 (30 microM) for up to 100 min reduced basal L(p) but did not attenuate the permeability increase induced by platelet activating factor (PAF) or bradykinin. These results show that toxin B-mediated reduction of endothelial barrier properties is due to inactivation of small GTPases other than RhoA. Rho proteins as well as RhoA-mediated contractile mechanisms are not involved in bradykinin- or PAF-induced hyperpermeability of intact microvessels.
Figures





References
-
- Aumailley M, Timpl R, Risau W. Differences in laminin fragment interactions of normal and transformed endothelial cells. Experimental Cell Research. 1991;196:177–183. - PubMed
-
- Busch C, Aktories K. Microbial toxins and the glycosylation of Rho family GTPases. Current Opinion in Structural Biology. 2000;10:528–535. - PubMed
-
- Carbajal JM, Gratrix ML, Yu CH, Schaeffer RC., Jr ROCK mediates thrombin's endothelial barrier dysfunction. American Journal of Physiology. 2000;279:C195–204. - PubMed
-
- Carbajal JM, Schaeffer RC., Jr RhoA inactivation enhances endothelial barrier function. American Journal of Physiology. 1999;277:C955–964. - PubMed
-
- Drenckhahn D, Ness W. The endothelial contractile cytoskeleton. In: Born GVR, Schwartz CJ, editors. Vascular Endothelium: Physiology, Pathology and Therapeutic Opportunities. Stuttgart: Schattauer; 1997. pp. 1–25.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

