Pharmacokinetics of a contraceptive patch (Evra/Ortho Evra) containing norelgestromin and ethinyloestradiol at four application sites
- PMID: 11851637
- PMCID: PMC1874289
- DOI: 10.1046/j.0306-5251.2001.01532.x
Pharmacokinetics of a contraceptive patch (Evra/Ortho Evra) containing norelgestromin and ethinyloestradiol at four application sites
Abstract
Aims: To determine the pharmacokinetic profile of norelgestromin (NGMN) and ethinyloestradiol (EE) following application of the contraceptive patch, Evra/Ortho Evra, at each of four anatomic sites (abdomen, buttock, arm, and torso).
Methods: Thirty-seven healthy, nonpregnant women aged 20-45 years participated in this open-label, four-period crossover study. Subjects were randomized to one of four treatment (site of application) sequences. Each patch was worn for 7 days, with a 1 month washout between treatments. Blood samples were collected before and at various times up to 240 h after application of each patch. Serum samples were assayed for NGMN and EE by validated methods.
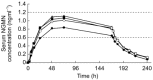
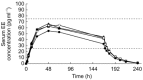
Results: The serum concentration reference ranges for NGMN and EE are 0.6-1.2 ng ml-1 and 25-75 pg ml-1, respectively, based on studies of the mean Cave of oral norgestimate 250 microg and EE 35 microg. For all application sites, mean concentrations of NGMN and EE remained within these ranges during the 7 day wear period. Absorption of NGMN and EE during patch application on the buttock, arm, and torso was equivalent. Absorption of NGMN and EE during patch application on the abdomen was approximately 20% less than observed for the other three sites, although mean serum concentrations were still within reference ranges. A previous study demonstrated therapeutic equivalence of patches worn on the abdomen vs other sites.
Conclusions: Serum concentrations of NGMN and EE from the contraceptive patch remain within the reference ranges throughout the 7 day wear period, regardless of the site of application (abdomen, buttock, arm, or torso).
Figures
References
-
- Fu H, Darroch JE, Haas T, Ranjit N. Contraceptive failure rates; new estimates from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999;31:56–63. - PubMed
-
- Trussell J, Vaughan B. Contraceptive failure, method-related discontinuation and resumption of use: results from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999;31:64–72. 93. - PubMed
-
- Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Stewart F, editors. Contraceptive Technology. Seventeenth edition. New York: Ardent Media, Inc; 1998. pp. 779–844.
-
- Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30(24–29):46. - PubMed
-
- McGuire JL, Phillips A, Hahn D, Tolman EL, Flor S, Kafrissen ME. Pharmacologic and pharmacokinetic characteristics of norgestimate and its metabolites. Am J Obstet Gynecol. 1990;163:2127–2131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

