Dose-effect relationship for several coagulation markers during administration of the direct thrombin inhibitor S 18326 in healthy subjects
- PMID: 11851638
- PMCID: PMC1874285
- DOI: 10.1046/j.0306-5251.2001.01534.x
Dose-effect relationship for several coagulation markers during administration of the direct thrombin inhibitor S 18326 in healthy subjects
Abstract
Aims: We conducted a phase I placebo-controlled trial with two i.v. doses (0.5 mg h-1 and 3 mg h-1) of S 18326, a selective thrombin inhibitor that interacts with the catalytic site of thrombin, with the aim to study the relationships between increasing plasma levels of S 18326 and changes in coagulation tests and thrombin generation markers.
Methods: Thirty-six healthy male volunteers were divided into three groups. In each group, 10 volunteers were randomly assigned to receive S 18326 and two to receive a placebo. Following a bolus of 4.5 mg, doses were 0.5 mg h-1 in the first group and 3 mg h-1 in the two other groups, administered as an i.v. infusion for 24 h. Blood was drawn repeatedly up to 36 h after the bolus, and tested for the activated clotting time (ACT) and activated partial thromboplastin time (APTT). The APTT reagent was chosen among five commercial reagents to yield a linear increase in the clotting time among possible therapeutic S 18326 concentrations in vitro. To accurately measure the thrombin-inhibiting effects of low doses of S 18326 (< 0.5 microm), we developed a specific chromogenic assay. We also measured F1 + 2 prothrombin fragment levels to assess the effect of S 18326 on thrombin generation in vivo.
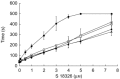
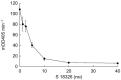
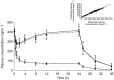
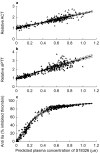
Results: A two-compartment pharmacokinetic model was fitted to the S 18326 plasma concentration vs time data by using population pharmacokinetic methods. Results of the pharmacodynamic-pharmacokinetic relationships showed that both the ACT and APTT methods yielded a linear increase according to the S 18326 concentration measured using a highly sensitive analytical method. At the end of infusion, ACT was prolonged 1.20 and 1.95-fold in the 0.5 mg h-1 and the 3 mg h-1 groups, respectively, and APTT was prolonged 1.27 and 2.75-fold. Thrombin inhibition plateaued above 0.5 microm of S 18326 according to an Emax model, confirming that the test was highly sensitive. F1 + 2 levels fell significantly after the 24 h S 18326 infusion (0.83 nm to 0.6 nm and 0.80 nm to 0.44 nm in the 0.5 mg h-1 and the 3 mg h-1 groups, respectively), but remained stable after the placebo infusion.
Conclusions: Our results support specific monitoring of the thrombin inhibitor S 18326 with ACT and APTT to establish the safety range of the drug in further studies. Moreover, the fall in F1 + 2 prothrombin fragments suggests that S 18326 effectively reduces the retroactivation of factors V and VIII by thrombin.
Figures
Similar articles
-
Recombinant hirudin as a periprocedural antithrombotic in coronary angioplasty for unstable angina pectoris.Eur Heart J. 1996 Aug;17(8):1207-15. doi: 10.1093/oxfordjournals.eurheartj.a015038. Eur Heart J. 1996. PMID: 8869862 Clinical Trial.
-
Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate.Thromb Haemost. 2012 May;107(5):985-97. doi: 10.1160/TH11-11-0804. Epub 2012 Mar 22. Thromb Haemost. 2012. PMID: 22438031
-
Transition from argatroban to oral anticoagulation with phenprocoumon or acenocoumarol: effects on prothrombin time, activated partial thromboplastin time, and Ecarin Clotting Time.Thromb Haemost. 2004 Jun;91(6):1137-45. doi: 10.1160/TH03-12-0794. Thromb Haemost. 2004. PMID: 15175800 Clinical Trial.
-
Dabigatran etexilate--a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity.Thromb Haemost. 2010 Jun;103(6):1116-27. doi: 10.1160/TH09-11-0758. Epub 2010 Mar 29. Thromb Haemost. 2010. PMID: 20352166 Review.
-
[Monitoring of Oral Thrombin Inhibitor].Rinsho Byori. 2014 Oct;62(10):958-64. Rinsho Byori. 2014. PMID: 27526541 Review. Japanese.
References
-
- Markwardt F. The development of hirudin as an antithrombotic agent. Thromb Res. 1994;74:1–23. - PubMed
-
- Rupin A, Mennecier Ph, de Nanteuil G, Laubie M, Verbeuren TJ. A screening procedure to evaluate the anticoagulant activity and the kinetic behaviour of direct thrombin inhibitors. Thromb Res. 1995;78:217–225. - PubMed
-
- Elg M, Gustafsson D, Deinum J. The importance of enzyme inhibition kinetics for the effect of thrombin inhibitors in a rat model of arterial thrombosis. Thromb Haemost. 1997;78:1286–1292. - PubMed
-
- Rupin A, Mennecier P, Lila C, de Nanteuil G, Verbeuren TJ. Selection of S 18326 as a new potent and selective boronic acid direct thrombin inhibitor. Thromb Haemost. 1997;78:1221–1227. - PubMed
-
- Stone SR, Le Bonniec BF. Inhibitory mechanism of serpins. Identification of steps involving the active-site serine residue of the protease. J Mol Biol. 1997;265:344–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

