Ketopremithramycins and ketomithramycins, four new aureolic acid-type compounds obtained upon inactivation of two genes involved in the biosynthesis of the deoxysugar moieties of the antitumor drug mithramycin by Streptomyces argillaceus, reveal novel insights into post-PKS tailoring steps of the mithramycin biosynthetic pathway
- PMID: 11853433
- PMCID: PMC4480631
- DOI: 10.1021/ja0105156
Ketopremithramycins and ketomithramycins, four new aureolic acid-type compounds obtained upon inactivation of two genes involved in the biosynthesis of the deoxysugar moieties of the antitumor drug mithramycin by Streptomyces argillaceus, reveal novel insights into post-PKS tailoring steps of the mithramycin biosynthetic pathway
Abstract
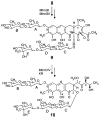
Mithramycin is an aureolic acid-type antimicrobial and antitumor agent produced by Streptomyces argillaceus. Modifying post-polyketide synthase (PKS) tailoring enzymes involved in the production of mithramycin is an effective way of gaining further information regarding the late steps of its biosynthetic pathway. In addition, new "unnatural" natural products of the aureolic acid-type class are likely to be produced. The role of two such post-PKS tailoring enzymes, encoded by mtmC and mtmTIII, was investigated, and four novel aureolic acid class drugs, two premithramycin-type molecules and two mithramycin derivatives, were isolated from mutant strains constructed by insertional gene inactivation of either of these two genes. From data bank comparisons, the corresponding proteins MtmC and MtmTIII were believed to act as a C-methyltransferase involved in the production of the D-mycarose (sugar E) of mithramycin and as a ketoreductase seemingly involved in the biosynthesis of the mithramycin aglycon, respectively. However, gene inactivation and analysis of the accumulated products revealed that both genes encode enzymes participating in the biosynthesis of the D-mycarose building block. Furthermore, the inactivation of MtmC seems to affect the ketoreductase responsible for 4-ketoreduction of sugar C, a D-olivose. Instead of obtaining premithramycin and mithramycin derivatives with a modified E-sugar upon inactivation of mtmC, compounds were obtained that completely lack the E-sugar moiety and that possess an unexpected 4-ketosugar moiety instead of the D-olivose at the beginning of the lower deoxysaccharide chain. The inactivation of mtmTIII led to the accumulation of 4E-ketomithramycin, showing that this ketoreductase is responsible for the 4-ketoreduction of the D-mycarose moiety. The new compounds of the mutant strains, 4A-ketopremithramycin A2, 4A-keto-9-demethylpremithramycin A2, 4C-keto-demycarosylmithramycin, and 4E-ketomithramycin, indicate surprising substrate flexibility of post-PKS enzymes of the mithramycin biosynthetic pathway. Although the glycosyltransferase responsible for the attachment of D-mycarose cannot transfer the unmethylated sugar to the existing lower disaccharide chain, it can transfer the 4-ketoform of sugar E. In addition, the glycosyltransferase MtmGIV, which is responsible for the linkage of sugar C, is also able to transfer an activated 4-ketosugar. The oxygenase MtmOIV, normally responsible for the oxidative cleavage of the tetracyclic premithramycin B into the tricyclic immediate precursor of mithramycin, can act on a substrate analogue with a modified or even incomplete trisaccharide chain. The same is true for glycosyltransferases MtmGI and MtmGII, both of which partake in the formation and attachment of the A-B disaccharide in mithramycin.
Figures



References
-
- Skarbek JD, Speedie MK. Antitumor Antibiotics of the Aureolic Acid Group: Chromomycin A3, Mithramycin A, and Olivomycin A. In: Aszalos A, editor. Antitumor Compounds of Natural Origin. Vol. 1. CRC Press; Boca Raton, FL: 1981. pp. 191–235.
-
- Van Dyke MW, Dervan PB. Biochemistry. 1983;22:2373–2377. - PubMed
-
- Remers WA. The Chemistry of Antitumor Antibiotics. Vol. 1. Wiley-Inter-science; New York: 1979. pp. 133–175.
-
- Rohr J, Méndez C, Salas JA. Bioorg Chem. 1999;27:41–54.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

