Cloned Shiga toxin 2 B subunit induces apoptosis in Ramos Burkitt's lymphoma B cells
- PMID: 11854211
- PMCID: PMC127801
- DOI: 10.1128/IAI.70.3.1279-1286.2002
Cloned Shiga toxin 2 B subunit induces apoptosis in Ramos Burkitt's lymphoma B cells
Retraction in
-
Cloned Shiga toxin 2 B subunit induces apoptosis in Ramos Burkitt's lymphoma B cells.Infect Immun. 2003 Aug;71(8):4828. doi: 10.1128/IAI.71.8.4828.2003. Infect Immun. 2003. PMID: 12894765 Free PMC article. No abstract available.
Abstract
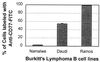
The Shiga toxins (Stx1 and Stx2), produced by Shigella dysenteriae type 1 and enterohemorrhagic Escherichia coli, consist of one A subunit and five B subunits. The Stx1 and Stx2 B subunits form a pentameric structure that binds to globotriaosylceramide (Gb3-Cer) receptors on eukaryotic cells and promotes endocytosis. The A subunit then inhibits protein biosynthesis, which triggers apoptosis in the affected cell. In addition to its Gb3-Cer binding activity, the data in the following report demonstrate that the Stx2 B pentamer induces apoptosis in Ramos Burkitt's lymphoma B cells independently of A subunit activity. Apoptosis was not observed in A subunit-free preparations of the Stx1 B pentamer which competitively inhibited Stx2 B pentamer-mediated apoptosis. The pancaspase inhibitor, Z-VAD-fmk, prevented apoptosis in Ramos cells exposed to the Stx2 B subunit, Stx1 or Stx2. Brefeldin A, an inhibitor of the Golgi transport system, also prevented Stx2 B subunit-mediated apoptosis. These observations suggest that the Stx2 B subunit must be internalized, via Gb3-Cer receptors, to induce Ramos cell apoptosis. Moreover, unlike the two holotoxins, Stx2 B subunit-mediated apoptosis does not involve inhibition of protein biosynthesis. This study provides further insight into the pathogenic potential of this family of potent bacterial exotoxins.
Figures



Similar articles
-
Escherichia coli shiga-like toxins induce apoptosis and cleavage of poly(ADP-ribose) polymerase via in vitro activation of caspases.Infect Immun. 2002 Aug;70(8):4669-77. doi: 10.1128/IAI.70.8.4669-4677.2002. Infect Immun. 2002. PMID: 12117981 Free PMC article.
-
Immunoprophylactic potential of cloned Shiga toxin 2 B subunit.J Infect Dis. 2001 Feb 1;183(3):435-43. doi: 10.1086/318080. Epub 2000 Dec 27. J Infect Dis. 2001. PMID: 11133375
-
Shiga toxins from enterohemorrhagic Escherichia coli and anti-GB3 antibody as novel agents against triple negative breast cancer.Chem Biol Interact. 2025 Oct 8;419:111639. doi: 10.1016/j.cbi.2025.111639. Epub 2025 Jul 4. Chem Biol Interact. 2025. PMID: 40617562
-
Construction of a novel bioluminescent reporter system for investigating Shiga toxin expression of enterohemorrhagic Escherichia coli.Gene. 2011 Jun 1;478(1-2):1-10. doi: 10.1016/j.gene.2011.01.006. Epub 2011 Jan 22. Gene. 2011. PMID: 21262333 Review.
-
Escherichia coli Shiga toxin.J Nat Toxins. 2000 Aug;9(3):299-313. J Nat Toxins. 2000. PMID: 10994531 Review.
Cited by
-
Oral administration of Shiga toxin-producing Escherichia coli induces intestinal and systemic specific immune response in mice.Med Microbiol Immunol. 2014 Jun;203(3):145-54. doi: 10.1007/s00430-013-0325-y. Epub 2014 Jan 8. Med Microbiol Immunol. 2014. PMID: 24399245
-
Serum amyloid P component binding to Shiga toxin 2 requires both a subunit and B pentamer.Infect Immun. 2003 Oct;71(10):6075-8. doi: 10.1128/IAI.71.10.6075-6078.2003. Infect Immun. 2003. PMID: 14500533 Free PMC article.
-
Activation of the Classical Mitogen-Activated Protein Kinases Is Part of the Shiga Toxin-Induced Ribotoxic Stress Response and May Contribute to Shiga Toxin-Induced Inflammation.Infect Immun. 2015 Oct 19;84(1):138-48. doi: 10.1128/IAI.00977-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26483408 Free PMC article.
-
Shiga toxin as a bacterial defense against a eukaryotic predator, Tetrahymena thermophila.J Bacteriol. 2009 Aug;191(16):5116-22. doi: 10.1128/JB.00508-09. Epub 2009 Jun 5. J Bacteriol. 2009. PMID: 19502393 Free PMC article.
-
Protection against Shiga Toxins.Toxins (Basel). 2017 Feb 3;9(2):44. doi: 10.3390/toxins9020044. Toxins (Basel). 2017. PMID: 28165371 Free PMC article. Review.
References
-
- Acheson, D. W., M. Jacewicz, A. V. Kane, A. Donohue-Rolfe, and G. T. Keusch. 1993. One-step high-yield affinity purification of shiga-like toxin II variants and quantitation using enzyme linked immunosorbent assays. Microb. Pathog. 14:57-66. - PubMed
-
- Arab, S., M. Murakami, P. Dirks, B. Boyd, S. L. Hubbard, C. A. Lingwood, and J. T. Rutka. 1998. Verotoxins inhibit the growth of and induce apoptosis in human astrocytoma cells. J. Neurooncol. 40:137-150. - PubMed
-
- Brown, J. E., M. A. Ussery, S. H. Leppla, and S. W. Rothman. 1980. Inhibition of protein synthesis by Shiga toxin: activation of the toxin and inhibition of peptide elongation. FEBS Lett. 117:84-88. - PubMed
-
- Chinnaiyan, A. M., K. O'Rourke, M. Tewari, and V. M. Dixit. 1995. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81:505-512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

