Control of experimental Trypanosoma brucei infections occurs independently of lymphotoxin-alpha induction
- PMID: 11854219
- PMCID: PMC127790
- DOI: 10.1128/IAI.70.3.1342-1351.2002
Control of experimental Trypanosoma brucei infections occurs independently of lymphotoxin-alpha induction
Abstract
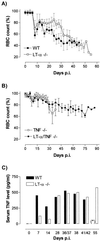
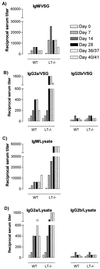
Trypanosome infections are marked by severe pathological features, including anemia, splenomegaly, and suppression of T-cell proliferation. We have used lymphotoxin-alpha-deficient (LT-alpha(-/-)) mice, as well as LT-alpha-tumor necrosis factor-double-deficient (LT-alpha(-/-) TNF(-/-)) mice, to analyze the contributions of these related cytokines in both induction of trypanosomosis-associated immunopathology and infection control. Moreover, as the cytokine-deficient mice used have no detectable lymph nodes and lack germinal-center formation upon immune stimulation, we have analyzed the functional importance of both the lymph nodes and spleen during experimental Trypanosoma brucei infections. First, we show that the absence of LT-alpha does not significantly alter early trypanosomosis development or pathology but does result in better control of late-stage parasitemia levels and slightly prolonged survival. This increased survival of infected LT-alpha(-/-) mice coincides with the appearance of increased chronic-stage anti-trypanosome immunoglobulin M (IgM)-IgG2a serum titers that are generated in the absence of functional peripheral lymphoid tissue and do not require germinal-center formation. Second, we show that splenectomized mice control their parasitemia to the same extent as fully immune-competent littermates. Finally, using LT-alpha(-/-) TNF(-/-) double-deficient mice, we show that in these mice T. brucei infections are very well controlled during the chronic infection stage and that infection-induced pathology is minimized. Together, these findings indicate that while increased IgM-IgG2a anti-trypanosome antibody titers (generated in the absence of LT-alpha, peripheral lymph nodes, and germinal-center formation) coincide with improved parasitemia control, it is TNF that has a major impact on trypanosomosis-associated immunopathology.
Figures
Similar articles
-
Influence of the Draining Lymph Nodes and Organized Lymphoid Tissue Microarchitecture on Susceptibility to Intradermal Trypanosoma brucei Infection.Front Immunol. 2020 Jun 3;11:1118. doi: 10.3389/fimmu.2020.01118. eCollection 2020. Front Immunol. 2020. PMID: 32582198 Free PMC article.
-
Tumor necrosis factor alpha is a key mediator in the regulation of experimental Trypanosoma brucei infections.Infect Immun. 1999 Jun;67(6):3128-32. doi: 10.1128/IAI.67.6.3128-3132.1999. Infect Immun. 1999. PMID: 10338530 Free PMC article.
-
Susceptibility of TNF-alpha-deficient mice to Trypanosoma congolense is not due to a defective antibody response.Acta Trop. 2004 Nov-Dec;92(3):193-203. doi: 10.1016/j.actatropica.2004.05.015. Acta Trop. 2004. PMID: 15533287
-
Alternative versus classical macrophage activation during experimental African trypanosomosis.Int J Parasitol. 2001 May 1;31(5-6):575-87. doi: 10.1016/s0020-7519(01)00170-9. Int J Parasitol. 2001. PMID: 11334945 Review.
-
Lymphotoxin-alpha-deficient and TNF receptor-I-deficient mice define developmental and functional characteristics of germinal centers.Immunol Rev. 1997 Apr;156:137-44. doi: 10.1111/j.1600-065x.1997.tb00965.x. Immunol Rev. 1997. PMID: 9176705 Review.
Cited by
-
Influence of the Draining Lymph Nodes and Organized Lymphoid Tissue Microarchitecture on Susceptibility to Intradermal Trypanosoma brucei Infection.Front Immunol. 2020 Jun 3;11:1118. doi: 10.3389/fimmu.2020.01118. eCollection 2020. Front Immunol. 2020. PMID: 32582198 Free PMC article.
-
Salivarian Trypanosomosis: A Review of Parasites Involved, Their Global Distribution and Their Interaction With the Innate and Adaptive Mammalian Host Immune System.Front Immunol. 2018 Oct 2;9:2253. doi: 10.3389/fimmu.2018.02253. eCollection 2018. Front Immunol. 2018. PMID: 30333827 Free PMC article. Review.
-
Clinical chemistry of congenic mice with quantitative trait loci for predicted responses to Trypanosoma congolense infection.Infect Immun. 2009 Sep;77(9):3948-57. doi: 10.1128/IAI.00658-09. Epub 2009 Jul 13. Infect Immun. 2009. PMID: 19596769 Free PMC article.
-
VSIG4-Expressing Macrophages Contribute to Antiparasitic and Antimetastatic Responses in the Peritoneal Cavity.Eur J Immunol. 2025 May;55(5):e202551804. doi: 10.1002/eji.202551804. Eur J Immunol. 2025. PMID: 40346775 Free PMC article.
-
The lymphatic system favours survival of a unique T. brucei population.Biol Open. 2023 Nov 15;12(11):bio059992. doi: 10.1242/bio.059992. Epub 2023 Nov 9. Biol Open. 2023. PMID: 37870927 Free PMC article.
References
-
- Assoku, R., I. Tizard, and K. Neilsen. 1977. Free fatty acids, complement activation, and polyclonal B-cell stimulation as factors in the immunopathogenesis of African trypanosomiasis. Lancet ii:956-959. - PubMed
-
- Beschin, A., L. Brys, S. Magez, M. Radwanska, and P. De Baetselier. 1998. Trypanosoma brucei infection elicits nitric oxide-dependent and nitric oxide-independent suppressive mechanisms. J. Leukoc. Biol. 63:429-439. - PubMed
-
- Cross, G. 1990. Cellular and genetic aspects on antigenic variation in trypanosomes. Annu. Rev. Immunol. 8:83-110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

