The sortase SrtA of Listeria monocytogenes is involved in processing of internalin and in virulence
- PMID: 11854224
- PMCID: PMC127754
- DOI: 10.1128/IAI.70.3.1382-1390.2002
The sortase SrtA of Listeria monocytogenes is involved in processing of internalin and in virulence
Abstract
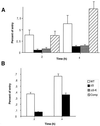
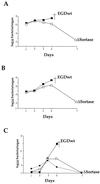
Listeria monocytogenes is an intracellular gram-positive human pathogen that invades eucaryotic cells. Among the surface-exposed proteins playing a role in this invasive process, internalin belongs to the family of LPXTG proteins, which are known to be covalently linked to the bacterial cell wall in gram-positive bacteria. Recently, it has been shown in Staphylococcus aureus that the covalent anchoring of protein A, a typical LPXTG protein, is due to a cysteine protease, named sortase, required for bacterial virulence. Here, we identified in silico from the genome of L. monocytogenes a gene, designated srtA, encoding a sortase homologue. The role of this previously unknown sortase was studied by constructing a sortase knockout mutant. Internalin was used as a reporter protein to study the effects of the srtA mutation on cell wall anchoring of this LPXTG protein in L. monocytogenes. We show that the srtA mutant (i) is affected in the display of internalin at the bacterial surface, (ii) is significantly less invasive in vitro, and (iii) is attenuated in its virulence in the mouse. These results demonstrate that srtA of L. monocytogenes acts as a sortase and plays a role in the pathogenicity.
Figures


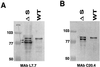
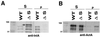
Similar articles
-
Sortase B, a new class of sortase in Listeria monocytogenes.J Bacteriol. 2004 Apr;186(7):1972-82. doi: 10.1128/JB.186.7.1972-1982.2004. J Bacteriol. 2004. PMID: 15028680 Free PMC article.
-
Inactivation of the srtA gene in Listeria monocytogenes inhibits anchoring of surface proteins and affects virulence.Mol Microbiol. 2002 Feb;43(4):869-81. doi: 10.1046/j.1365-2958.2002.02798.x. Mol Microbiol. 2002. PMID: 11929538
-
LPXTG protein InlJ, a newly identified internalin involved in Listeria monocytogenes virulence.Infect Immun. 2005 Oct;73(10):6912-22. doi: 10.1128/IAI.73.10.6912-6922.2005. Infect Immun. 2005. PMID: 16177371 Free PMC article.
-
Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus.Mol Microbiol. 2001 Jun;40(5):1049-57. doi: 10.1046/j.1365-2958.2001.02411.x. Mol Microbiol. 2001. PMID: 11401711 Review.
-
Sortase A (SrtA) inhibitors as an alternative treatment for superbug infections.Drug Discov Today. 2021 Sep;26(9):2164-2172. doi: 10.1016/j.drudis.2021.03.019. Epub 2021 Mar 27. Drug Discov Today. 2021. PMID: 33781954 Review.
Cited by
-
3D-QSAR Studies of 1,2,4-Oxadiazole Derivatives as Sortase A Inhibitors.Biomed Res Int. 2021 Dec 6;2021:6380336. doi: 10.1155/2021/6380336. eCollection 2021. Biomed Res Int. 2021. PMID: 34912894 Free PMC article.
-
Discovery and structure-activity relationship analysis of Staphylococcus aureus sortase A inhibitors.Bioorg Med Chem. 2009 Oct 15;17(20):7174-85. doi: 10.1016/j.bmc.2009.08.067. Epub 2009 Sep 6. Bioorg Med Chem. 2009. PMID: 19781950 Free PMC article.
-
Synthesis and structure activity relationship studies of novel Staphylococcus aureus Sortase A inhibitors.Eur J Med Chem. 2010 Sep;45(9):3752-61. doi: 10.1016/j.ejmech.2010.05.024. Epub 2010 May 20. Eur J Med Chem. 2010. PMID: 20541848 Free PMC article.
-
Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria.Microbiol Mol Biol Rev. 2006 Mar;70(1):192-221. doi: 10.1128/MMBR.70.1.192-221.2006. Microbiol Mol Biol Rev. 2006. PMID: 16524923 Free PMC article. Review.
-
Home Alone: Elimination of All but One Alternative Sigma Factor in Listeria monocytogenes Allows Prediction of New Roles for σB.Front Microbiol. 2017 Oct 11;8:1910. doi: 10.3389/fmicb.2017.01910. eCollection 2017. Front Microbiol. 2017. PMID: 29075236 Free PMC article.
References
-
- Berche, P. 1995. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb. Pathog. 18:323-336. - PubMed
-
- Braun, L., S. Dramsi, P. Dehoux, H. Bierne, G. Lindahl, and P. Cossart. 1997. InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol. Microbiol. 25:285-294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

