Osmolarity and pH growth conditions regulate fim gene transcription and type 1 pilus expression in uropathogenic Escherichia coli
- PMID: 11854225
- PMCID: PMC127777
- DOI: 10.1128/IAI.70.3.1391-1402.2002
Osmolarity and pH growth conditions regulate fim gene transcription and type 1 pilus expression in uropathogenic Escherichia coli
Abstract
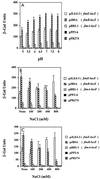
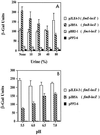
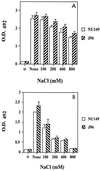
A comparative study was performed to determine the effects of pH, osmolarity, and human urine on the transcription of several fim genes, as well as the overall expression of type 1 pili. Several fim-lacZYA fusions were constructed on single-copy plasmids to test a range of pHs and a range of osmolarities. Growth in acidic medium slightly reduced expression from all of the fim promoters (fimA, fimB, and fimE). Increased osmolarity in neutral-pH medium repressed fimA and fimB transcription by approximately 50% when 400 mM NaCl was used and nearly threefold when 800 mM NaCl was used, whereas fimE transcription rose slightly as the osmolarity increased. This effect was more pronounced in high-osmolarity acidic media; fimB and fimA expression decreased fivefold in growth media containing 800 mM NaCl compared to expression in growth media without added NaCl. Moreover, fimE expression doubled under the same high-osmolarity conditions compared to expression in a low-osmolarity acidic environment. When a fimB-lacZ or fimE-lacZ fusion was inserted into the chromosome of strain AAEC189, fimE expression changed slightly as the osmolarity increased, but fimB expression decreased by 50% in a low-pH high-osmolarity environment. When strain AAEC189 with either a plasmid-borne fimB-lacZ fusion or a plasmid-borne fimE-lacZ fusion was grown in human urine, similar changes in the levels of fimB and fimE expression were observed. Limiting-dilution reverse transcription-PCR confirmed that these changes in fim expression occurred in clinical isolates of uropathogenic Escherichia coli grown in media with different pHs and different osmolarities. Furthermore, the invertible switch region in uropathogenic strain NU149 shifted from favoring the phase-on position in a neutral-pH low-osmolarity environment to favoring the phase-off position in a low-pH high-osmolarity environment. Results obtained with an ompR mutant strain demonstrated that fimB expression was derepressed and that OmpR may neutralize repression by an acid response regulator of fimE expression in a low-pH environment. In addition, H-NS was verified to be important in regulation of fimB, but it had only a slight effect on fimE under the specific pH and osmotic growth conditions tested. Enzyme immunoassays with anti-type 1 pilus antibody and hemagglutination assays showed that fewer type 1 pili were detected with cells in a low-pH high-osmolarity environment. Together, these observations demonstrate that a combination of low pH and high osmolarity regulates the transcription of fim genes, which favors a shift in the invertible element to the phase-off orientation and a loss of type 1 pilus expression. Taken together, our data suggest that the environmental cues that we tested may regulate expression of type 1 pili in specific in vivo niches, such as murine kidneys and possibly human kidneys.
Figures

Similar articles
-
OmpR regulation of the uropathogenic Escherichia coli fimB gene in an acidic/high osmolality environment.Microbiology (Reading). 2013 Feb;159(Pt 2):316-327. doi: 10.1099/mic.0.059386-0. Epub 2012 Nov 22. Microbiology (Reading). 2013. PMID: 23175504 Free PMC article.
-
Regulation of type 1 fimbriae by unlinked FimB- and FimE-like recombinases in uropathogenic Escherichia coli strain CFT073.Infect Immun. 2006 Feb;74(2):1072-83. doi: 10.1128/IAI.74.2.1072-1083.2006. Infect Immun. 2006. PMID: 16428754 Free PMC article.
-
The Pai-associated leuX specific tRNA5(Leu) affects type 1 fimbriation in pathogenic Escherichia coli by control of FimB recombinase expression.Mol Microbiol. 1997 Sep;25(5):871-82. doi: 10.1111/j.1365-2958.1997.mmi517.x. Mol Microbiol. 1997. PMID: 9364913
-
The regulation of pap and type 1 fimbriation in Escherichia coli.Adv Microb Physiol. 2001;45:1-49. doi: 10.1016/s0065-2911(01)45001-6. Adv Microb Physiol. 2001. PMID: 11450107 Review.
-
Biogenesis of Type V pili.Microbiol Immunol. 2020 Oct;64(10):643-656. doi: 10.1111/1348-0421.12838. Epub 2020 Sep 9. Microbiol Immunol. 2020. PMID: 32816331 Review.
Cited by
-
The Flagellar Transcriptional Regulator FtcR Controls Brucella melitensis 16M Biofilm Formation via a betI-Mediated Pathway in Response to Hyperosmotic Stress.Int J Mol Sci. 2022 Aug 31;23(17):9905. doi: 10.3390/ijms23179905. Int J Mol Sci. 2022. PMID: 36077302 Free PMC article.
-
Innate Bacteriostatic Mechanisms Defend the Urinary Tract.Annu Rev Physiol. 2022 Feb 10;84:533-558. doi: 10.1146/annurev-physiol-052521-121810. Epub 2021 Nov 15. Annu Rev Physiol. 2022. PMID: 34780258 Free PMC article. Review.
-
Identification of functions linking quorum sensing with biofilm formation in Burkholderia cenocepacia H111.Microbiologyopen. 2012 Jun;1(2):225-42. doi: 10.1002/mbo3.24. Microbiologyopen. 2012. PMID: 22950027 Free PMC article.
-
Function and expression of an N-acetylneuraminic acid-inducible outer membrane channel in Escherichia coli.J Bacteriol. 2005 Mar;187(6):1959-65. doi: 10.1128/JB.187.6.1959-1965.2005. J Bacteriol. 2005. PMID: 15743943 Free PMC article.
-
HbiF regulates type 1 fimbriation independently of FimB and FimE.Infect Immun. 2006 Jul;74(7):4039-47. doi: 10.1128/IAI.02058-05. Infect Immun. 2006. PMID: 16790777 Free PMC article.
References
-
- Asscher, A. W., M. Sussman, W. E. Waters, R. H. Davis, and S. Chick. 1966. Urine as a medium for bacterial growth. Lancet ii:1037-1041. - PubMed
-
- Atlung, T., and H. Ingmer. 1997. H-NS: a modulator of environmentally regulated gene expression. Mol. Microbiol. 24:7-17. - PubMed
-
- Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

