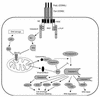
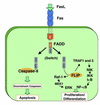
Death receptors couple to both cell proliferation and apoptosis
- PMID: 11854313
- PMCID: PMC150882
- DOI: 10.1172/JCI15077
Death receptors couple to both cell proliferation and apoptosis
Figures
References
-
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. - PubMed
-
- Krammer PH. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163–210. - PubMed
-
- Thornberry NA, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. - PubMed
-
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. - PubMed
-
- Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

