Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes
- PMID: 11854325
- PMCID: PMC150879
- DOI: 10.1172/JCI14550
Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes
Abstract
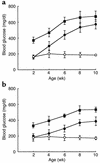
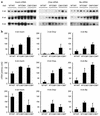
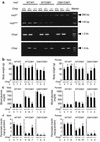
Overload of pancreatic beta cells in conditions such as hyperglycemia, obesity, and long-term treatment with sulfonylureas leads to beta cell exhaustion and type 2 diabetes. Because beta cell mass declines under these conditions, apparently as a result of apoptosis, we speculated that overload kills beta cells as a result of endoplasmic reticulum (ER) stress. The Akita mouse, which carries a conformation-altering missense mutation (Cys96Tyr) in Insulin 2, likewise exhibits hyperglycemia and a reduced beta cell mass. In the development of diabetes in Akita mice, mRNAs for the ER chaperone Bip and the ER stress-associated apoptosis factor Chop were induced in the pancreas. Overexpression of the mutant insulin in mouse MIN6 beta cells induced Chop expression and led to apoptosis. Targeted disruption of the Chop gene delayed the onset of diabetes in heterozygous Akita mice by 8-10 weeks. We conclude that ER overload in beta cells causes ER stress and leads to apoptosis via Chop induction. Our findings suggest a new therapeutic approach for preventing the onset of diabetes by inhibiting Chop induction or by increasing chaperone capacity in the ER.
Figures



Comment in
-
Proteotoxicity in the endoplasmic reticulum: lessons from the Akita diabetic mouse.J Clin Invest. 2002 Feb;109(4):443-5. doi: 10.1172/JCI15020. J Clin Invest. 2002. PMID: 11854314 Free PMC article. Review. No abstract available.
References
-
- Stefan Y, et al. Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes. 1982;31:694–700. - PubMed
-
- Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–371. - PubMed
-
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. - PubMed
-
- Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. - PubMed
-
- Travers KJ, et al. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

