Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm
- PMID: 11854401
- PMCID: PMC65638
- DOI: 10.1091/mbc.01-06-0308
Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm
Abstract
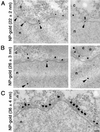
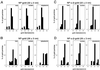
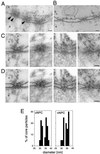
Bidirectional transport of macromolecules between the nucleus and the cytoplasm occurs through the nuclear pore complexes (NPCs) by a signal-mediated mechanism that is directed by targeting signals (NLSs) residing on the transported molecules or "cargoes." Nuclear transport starts after interaction of the targeting signal with soluble cellular receptors. After the formation of the cargo-receptor complex in the cytosol, this complex crosses the NPC. Herein, we use gold particles of various sizes coated with cargo-receptor complexes to determine precisely how large macromolecules crossing the NPC by the signal-mediated transport mechanism could be. We found that cargo-receptor-gold complexes with diameter close to 39 nm could be translocated by the NPC. This implies that macromolecules much larger than the assumed functional NPC diameter of 26 nm can be transported into the karyoplasm. The physiological relevance of this finding was supported by the observation that intact nucleocapsids of human hepatitis B virus with diameters of 32 and 36 nm are able to cross the nuclear pore without disassembly.
Figures


References
-
- Baschong W, Wrigley NG. Small colloidal gold conjugated to Fab fragments or to immunoglobulin G as high-resolution labels for electron microscopy: a technical overview. J Electron Microsc Tech. 1990;14:313–323. - PubMed
-
- Catimel B, Teh T, Fontes MR, Jennings IG, Jans DA, Howlett GJ, Nice EC, Kobe B. Biophysical characterization of interactions involving importin-alpha during nuclear import. J Biol Chem. 2001;276:34189–34198. - PubMed
-
- Chook YM, Cingolani G, Conti E, Stewart M, Vetter I, Wittinghofer A. Pictures in cell biology. Structures of nuclear-transport components. Trends Cell Biol. 1999;9:310–311. - PubMed
-
- Cingolani G, Petosa C, Weis K, Muller CW. Structure of importin-beta bound to the IBB domain of importin-alpha. Nature. 1999;399:221–229. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

