Essential role of MCM proteins in premeiotic DNA replication
- PMID: 11854402
- PMCID: PMC65639
- DOI: 10.1091/mbc.01-11-0537
Essential role of MCM proteins in premeiotic DNA replication
Abstract
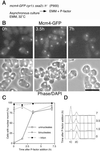
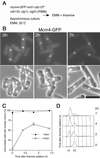
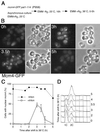
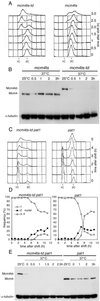
A critical event in eukaryotic DNA replication involves association of minichromosome maintenance (MCM2-7) proteins with origins, to form prereplicative complexes (pre-RCs) that are competent for initiation. The ability of mutants defective in MCM2-7 function to complete meiosis had suggested that pre-RC components could be irrelevant to premeiotic S phase. We show here that MCM2-7 proteins bind to chromatin in fission yeast cells preparing for meiosis and during premeiotic S phase in a manner suggesting they in fact are required for DNA replication in the meiotic cycle. This is confirmed by analysis of a degron mcm4 mutant, which cannot carry out premeiotic DNA replication. Later in meiosis, Mcm4 chromatin association is blocked between meiotic nuclear divisions, presumably accounting for the absence of a second round of DNA replication. Together, these results emphasize similarity between replication mechanisms in mitotic and meiotic cell cycles.
Figures


Similar articles
-
The role and regulation of the preRC component Cdc6 in the initiation of premeiotic DNA replication.Mol Biol Cell. 2004 May;15(5):2230-42. doi: 10.1091/mbc.e03-08-0617. Epub 2004 Mar 5. Mol Biol Cell. 2004. PMID: 15004237 Free PMC article.
-
MCM2-7 proteins are essential components of prereplicative complexes that accumulate cooperatively in the nucleus during G1-phase and are required to establish, but not maintain, the S-phase checkpoint.Mol Biol Cell. 2001 Nov;12(11):3658-67. doi: 10.1091/mbc.12.11.3658. Mol Biol Cell. 2001. PMID: 11694596 Free PMC article.
-
Mitotic replication initiation proteins are not required for pre-meiotic S phase.Nat Genet. 2000 Jul;25(3):263-8. doi: 10.1038/77015. Nat Genet. 2000. PMID: 10888871
-
Cdc6 and DNA replication: limited to humble origins.Bioessays. 1996 Nov;18(11):859-62. doi: 10.1002/bies.950181103. Bioessays. 1996. PMID: 8939063 Review.
-
MCM proteins in DNA replication.Annu Rev Biochem. 1999;68:649-86. doi: 10.1146/annurev.biochem.68.1.649. Annu Rev Biochem. 1999. PMID: 10872463 Review.
Cited by
-
DNA damage induces Cdt1 proteolysis in fission yeast through a pathway dependent on Cdt2 and Ddb1.EMBO Rep. 2006 Nov;7(11):1134-9. doi: 10.1038/sj.embor.7400827. Epub 2006 Oct 13. EMBO Rep. 2006. PMID: 17039252 Free PMC article.
-
The Cyclin-dependent kinase inhibitor Dacapo promotes genomic stability during premeiotic S phase.Mol Biol Cell. 2009 Apr;20(7):1960-9. doi: 10.1091/mbc.e08-09-0916. Epub 2009 Feb 11. Mol Biol Cell. 2009. PMID: 19211840 Free PMC article.
-
Hsk1 kinase is required for induction of meiotic dsDNA breaks without involving checkpoint kinases in fission yeast.Proc Natl Acad Sci U S A. 2006 May 23;103(21):8131-6. doi: 10.1073/pnas.0602498103. Epub 2006 May 12. Proc Natl Acad Sci U S A. 2006. PMID: 16698922 Free PMC article.
-
Fission yeast Cdc23/Mcm10 functions after pre-replicative complex formation to promote Cdc45 chromatin binding.Mol Biol Cell. 2003 Sep;14(9):3876-87. doi: 10.1091/mbc.e03-02-0090. Epub 2003 Jun 13. Mol Biol Cell. 2003. PMID: 12972571 Free PMC article.
-
Sequential steps in DNA replication are inhibited to ensure reduction of ploidy in meiosis.Mol Biol Cell. 2013 Mar;24(5):578-87. doi: 10.1091/mbc.E12-11-0825. Epub 2013 Jan 9. Mol Biol Cell. 2013. PMID: 23303250 Free PMC article.
References
-
- Adachi Y, Usukura J, Yanagida M. A globular complex formation by Nda1 and the other five members of the MCM protein family in fission yeast. Genes Cells. 1997;2:467–479. - PubMed
-
- Bähler J, Schuchert P, Grimm C, Kohli J. Synchronized meiosis and recombination in fission yeast: observations with pat1-114 diploid cells. Curr Genet. 1991;19:445–451. - PubMed
-
- Bähler J, Wu JQ, Longtine MS, Shah NG, McKenzie A, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. - PubMed
-
- Borde V, Goldman ASH, Lichten M. Direct coupling between meiotic DNA replication and recombination initiation. Science. 2000;290:806–809. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

