Secretion and assembly of zona pellucida glycoproteins by growing mouse oocytes microinjected with epitope-tagged cDNAs for mZP2 and mZP3
- PMID: 11854410
- PMCID: PMC65647
- DOI: 10.1091/mbc.01-09-0440
Secretion and assembly of zona pellucida glycoproteins by growing mouse oocytes microinjected with epitope-tagged cDNAs for mZP2 and mZP3
Abstract
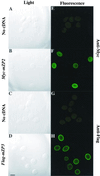
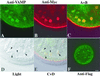
The zona pellucida (ZP) is a highly organized extracellular coat that surrounds all mammalian eggs. The mouse egg ZP is composed of three glycoproteins, called mZP1-3, that are synthesized, secreted, and assembled into a ZP exclusively by growing oocytes. Here, we microinjected epitope-tagged (Myc and Flag) cDNAs for mZP2 and mZP3 into the germinal vesicle (nucleus) of growing oocytes isolated from juvenile mice. Specific antibodies and laser scanning confocal microscopy were used to follow nascent, recombinant ZP glycoproteins in both permeabilized and nonpermeabilized oocytes. When such cDNAs were injected, epitope-tagged mZP2 (Myc-mZP2) and mZP3 (Flag-mZP3) were synthesized, packaged into large intracellular vesicles, and secreted by the vast majority of oocytes. Secreted glycoproteins were incorporated into only the innermost layer of the thickening ZP, and the amount of nascent glycoprotein in this region increased with increasing time of oocyte culture. Consistent with prior observations, the putative transmembrane domain at the C terminus of mZP2 and mZP3 was missing from nascent glycoprotein incorporated into the ZP. When the consensus furin cleavage site near the C terminus of mZP3 was mutated, such that it should not be cleaved by furin, secretion and assembly of mZP3 was reduced. On the other hand, mZP3 incorporated into the ZP lacked the transmembrane domain downstream of the mutated furin cleavage site, suggesting that some other protease(s) excised the domain. These results strongly suggest that nascent mZP2 and mZP3 are incorporated into only the innermost layer of the ZP and that excision of the C-terminal region of the glycoproteins is required for assembly into the oocyte ZP.
Figures







References
-
- Beebe SL, Leyton L, Burks D, Ishikawa M, Fuerst T, Dean J, Saling P. Recombinant mouse ZP3 inhibits sperm binding and induces the acrosome reaction. Dev Biol. 1992;151:48–54. - PubMed
-
- Bork P, Sander C. A large domain common to sperm receptors (ZP2 and ZP3) and TGF-β type III receptor. FEBS Lett. 1992;300:237–240. - PubMed
-
- Chalifour LE, Wirak DO, Hansen U, Wassarman PM, DePamphilis ML. Cis- and trans-acting sequences required for the expression of simian virus 40 genes in mouse oocytes. Genes Dev. 1987;1:1096–1106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

