Daughter cell assembly in the protozoan parasite Toxoplasma gondii
- PMID: 11854415
- PMCID: PMC65652
- DOI: 10.1091/mbc.01-06-0309
Daughter cell assembly in the protozoan parasite Toxoplasma gondii
Abstract
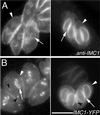
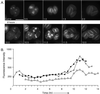
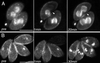
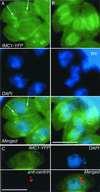
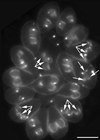
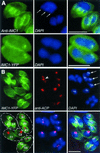
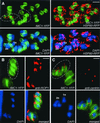
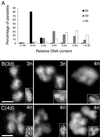
The phylum Apicomplexa includes thousands of species of obligate intracellular parasites, many of which are significant human and/or animal pathogens. Parasites in this phylum replicate by assembling daughters within the mother, using a cytoskeletal and membranous scaffolding termed the inner membrane complex. Most apicomplexan parasites, including Plasmodium sp. (which cause malaria), package many daughters within a single mother during mitosis, whereas Toxoplasma gondii typically packages only two. The comparatively simple pattern of T. gondii cell division, combined with its molecular genetic and cell biological accessibility, makes this an ideal system to study parasite cell division. A recombinant fusion between the fluorescent protein reporter YFP and the inner membrane complex protein IMC1 has been exploited to examine daughter scaffold formation in T. gondii. Time-lapse video microscopy permits the entire cell cycle of these parasites to be visualized in vivo. In addition to replication via endodyogeny (packaging two parasites at a time), T. gondii is also capable of forming multiple daughters, suggesting fundamental similarities between cell division in T. gondii and other apicomplexan parasites.
Figures

References
-
- Aikawa M. Plasmodium: the fine structure of malarial parasites. Exp Parasitol. 1971;30:284–320. - PubMed
-
- Azab M, Beverley JK. Schizogony of Toxoplasma gondii in tissue cultures. Z Parasitenkd. 1974;44:33–41. - PubMed
-
- Bannister LH, Hopkins JM, Fowler RE, Krishna S, Mitchell GH. A brief illustrated guide to the ultrastructure of Plasmodium falciparum asexual blood stages. Parasitol Today. 2000;16:427–433. - PubMed
-
- Bonhomme A, Pingret L, Pinon JM. Review: Toxoplasma gondii cellular invasion. Parassitologia. 1992;34:31–43. - PubMed
-
- Brockelman CR, Tan-Ariya P, Laovanitch R. Observation on complete schizogony of Plasmodium vivax in vitro. J Protozool. 1985;32:76–80. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

