Dictyostelium LvsB mutants model the lysosomal defects associated with Chediak-Higashi syndrome
- PMID: 11854420
- PMCID: PMC65657
- DOI: 10.1091/mbc.01-09-0454
Dictyostelium LvsB mutants model the lysosomal defects associated with Chediak-Higashi syndrome
Abstract
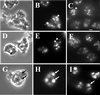
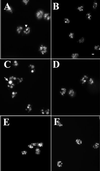
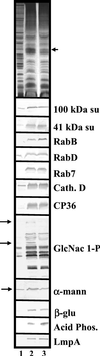
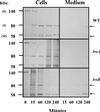
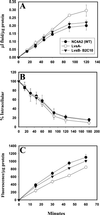
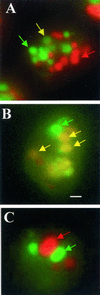
Chediak-Higashi syndrome is a genetic disorder caused by mutations in a gene encoding a protein named LYST in humans ("lysosomal trafficking regulator") or Beige in mice. A prominent feature of this disease is the accumulation of enlarged lysosome-related granules in a variety of cells. The genome of Dictyostelium discoideum contains six genes encoding proteins that are related to LYST/Beige in amino acid sequence, and disruption of one of these genes, lvsA (large volume sphere), results in profound defects in cytokinesis. To better understand the function of this family of proteins in membrane trafficking, we have analyzed mutants disrupted in lvsA, lvsB, lvsC, lvsD, lvsE, and lvsF. Of all these, only lvsA and lvsB mutants displayed interesting phenotypes in our assays. lvsA-null cells exhibited defects in phagocytosis and contained abnormal looking contractile vacuole membranes. Loss of LvsB, the Dictyostelium protein most similar to LYST/Beige, resulted in the formation of enlarged vesicles that by multiple criteria appeared to be acidic lysosomes. The rates of endocytosis, phagocytosis, and fluid phase exocytosis were normal in lvsB-null cells. Also, the rates of processing and the efficiency of targeting of lysosomal alpha-mannosidase were normal, although lvsB mutants inefficiently retained alpha-mannosidase, as well as two other lysosomal cysteine proteinases. Finally, results of pulse-chase experiments indicated that an increase in fusion rates accounted for the enlarged lysosomes in lvsB-null cells, suggesting that LvsB acts as a negative regulator of fusion. Our results support the notion that LvsB/LYST/Beige function in a similar manner to regulate lysosome biogenesis.
Figures





References
-
- Adam-Klages S, Adam D, Wiegmann K, Struve S, Kolanus W, Schneider-Mergener J, Kronke M. FAN, a novel WD-repeat protein, couples the p55 TNF-receptor to neutral sphingomyelinase. Cell. 1996;86:937–947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

