Differential effects of a centrally acting fatty acid synthase inhibitor in lean and obese mice
- PMID: 11854492
- PMCID: PMC122295
- DOI: 10.1073/pnas.042683699
Differential effects of a centrally acting fatty acid synthase inhibitor in lean and obese mice
Erratum in
- Proc Natl Acad Sci U S A 2002 May 28;99(11):7809
Abstract
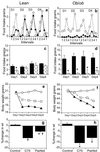
C75 is a potent inhibitor of fatty acid synthase that acts centrally to reduce food intake and body weight in mice; a single dose causes a rapid (>90%) decrease of food intake. These effects are associated with inhibition of fasting-induced up-regulation and down-regulation, respectively, of the expression of orexigenic (NPY and AgRP) and anorexigenic (POMC and CART) neuropeptide messages in the hypothalamus. Repeated administration of C75 at a submaximal level, however, differentially affected food intake of lean and obese mice. With lean mice, C75 suppressed food intake by approximately 50% and, with obese mice (ob/ob and dietary-induced obesity), by 85-95% during the first day of treatment. Lean mice, however, became tolerant/resistant to C75 over the next 2-5 days of treatment, with food intake returning to near normal and rebound hyperphagia occurring on cessation of treatment. In contrast, ob/ob obese mice responded to C75 with a >90% suppression of food intake throughout the same period with incipient tolerance becoming evident only after substantial weight loss had occurred. Dietary-induced obese mice exhibited intermediate behavior. In all cases, a substantial loss of body weight resulted. Pair-fed controls lost 24-50% less body weight than C75-treated mice, indicating that, in addition to suppressing food intake, C75 may increase energy expenditure. The decrease in body weight by ob/ob mice was due primarily to loss of body fat. In contrast to the short-term effects of C75 on "fasting-induced" changes of hypothalamic orexigenic and anorexigenic neuropeptide mRNAs, repeated administration of C75 either had the inverse or no effect as tolerance developed.
Figures
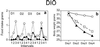


References
-
- Schwartz M W, Woods S C, Porte D, Jr, Seeley R J, Baskin D G. Nature (London) 2000;404:661–671. - PubMed
-
- Beck B. Nutrition. 2000;16:916–923. - PubMed
-
- Loftus T M, Jaworsky D E, Frehywot G L, Townsend C A, Ronnett G V, Lane M D, Kuhajda F P. Science. 2000;288:2379–2381. - PubMed
-
- Wakil S. Biochemistry. 1989;28:4523–4530. - PubMed
-
- Bouchard C. N Engl J Med. 2000;343:1888–1889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

