Functional studies on the role of the C-terminal domain of mammalian polo-like kinase
- PMID: 11854496
- PMCID: PMC122306
- DOI: 10.1073/pnas.042689299
Functional studies on the role of the C-terminal domain of mammalian polo-like kinase
Abstract
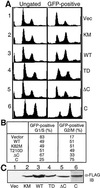
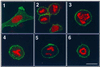
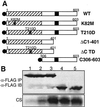
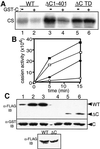
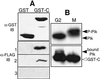
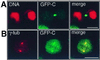
Mammalian polo-like kinase (Plk) acts at various stages in early and late mitosis. Plk is phosphorylated and activated in mitosis, and the proper subcellular localization of Plk is essential for mitotic regulation. We have observed that overexpression of the C-terminal domain of Plk is more effective than wild-type or kinase-defective Plk in causing mitotic delay or arrest. The specific activity of Plk with C-terminal deletions or substitution of aspartate for threonine-210 is increased severalfold relative to wild type. We show in this communication that the C-terminal domain can bind to full-length or the catalytic domain of Plk and inhibit its kinase activity, and that this binding is disrupted when threonine-210 is substituted with an aspartic acid residue. The C-terminal domain binds unphosphorylated Plk from G(2) arrested cells, but not phosphorylated Plk from mitotic cells. Green fluorescent protein-C-terminal Plk is localized at the centrosome and the midbody of transfected cells as shown previously for full-length enzyme. These and other data indicate that although the C terminus serves to regulate Plk kinase activity, the localization of the C terminus at the centrosome and other sites in transfected cells may block the correct localization of endogenous Plk.
Figures
Similar articles
-
Phosphorylation of threonine 210 and the role of serine 137 in the regulation of mammalian polo-like kinase.J Biol Chem. 2002 Nov 15;277(46):44115-20. doi: 10.1074/jbc.M202172200. Epub 2002 Aug 30. J Biol Chem. 2002. PMID: 12207013
-
Polo box domain of Plk3 functions as a centrosome localization signal, overexpression of which causes mitotic arrest, cytokinesis defects, and apoptosis.J Biol Chem. 2006 Apr 14;281(15):10577-82. doi: 10.1074/jbc.M513156200. Epub 2006 Feb 14. J Biol Chem. 2006. PMID: 16478733
-
Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate.J Biol Chem. 2003 Jul 11;278(28):25277-80. doi: 10.1074/jbc.C300126200. Epub 2003 May 8. J Biol Chem. 2003. PMID: 12738781
-
Molecular and enzoinformatics perspectives of targeting Polo-like kinase 1 in cancer therapy.Semin Cancer Biol. 2019 Jun;56:47-55. doi: 10.1016/j.semcancer.2017.11.004. Epub 2017 Nov 6. Semin Cancer Biol. 2019. PMID: 29122685 Review.
-
The Aurora kinases: role in cell transformation and tumorigenesis.Cancer Metastasis Rev. 2003 Dec;22(4):451-64. doi: 10.1023/a:1023789416385. Cancer Metastasis Rev. 2003. PMID: 12884918 Review.
Cited by
-
Distinct surfaces on Cdc5/PLK Polo-box domain orchestrate combinatorial substrate recognition during cell division.Sci Rep. 2020 Feb 25;10(1):3379. doi: 10.1038/s41598-020-60344-4. Sci Rep. 2020. PMID: 32099015 Free PMC article.
-
Mitosis-specific phosphorylation of Mis18α by Aurora B kinase enhances kinetochore recruitment of polo-like kinase 1.Oncotarget. 2017 Nov 27;9(2):1563-1576. doi: 10.18632/oncotarget.22707. eCollection 2018 Jan 5. Oncotarget. 2017. PMID: 29416714 Free PMC article.
-
PLK1 blockade enhances therapeutic effects of radiation by inducing cell cycle arrest at the mitotic phase.Sci Rep. 2015 Oct 27;5:15666. doi: 10.1038/srep15666. Sci Rep. 2015. PMID: 26503893 Free PMC article.
-
PP2A-Tws dephosphorylates Map205, is required for Polo localization to microtubules and promotes cytokinesis in Drosophila.Cell Div. 2024 Dec 28;19(1):36. doi: 10.1186/s13008-024-00141-x. Cell Div. 2024. PMID: 39732709 Free PMC article.
-
Serendipitous alkylation of a Plk1 ligand uncovers a new binding channel.Nat Chem Biol. 2011 Jul 17;7(9):595-601. doi: 10.1038/nchembio.614. Nat Chem Biol. 2011. PMID: 21765407 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

