Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors
- PMID: 11854531
- PMCID: PMC122373
- DOI: 10.1073/pnas.032423699
Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors
Abstract
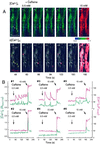
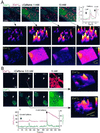
Propagation of cytosolic [Ca(2+)] ([Ca(2+)](c)) signals to the mitochondria is believed to be supported by a local communication between Ca(2+) release channels and adjacent mitochondrial Ca(2+) uptake sites, but the signaling machinery has not been explored at the level of elementary Ca(2+) release events. Here, we demonstrate that [Ca(2+)](c) sparks mediated by ryanodine receptors are competent to elicit miniature mitochondrial matrix [Ca(2+)] signals that we call "Ca(2+) marks." Ca(2+) marks are restricted to single mitochondria and typically last less than 500 ms. The decay of Ca(2+) marks relies on extrusion of Ca(2+) from the mitochondria through the Ca(2+) exchanger, whereas [Ca(2+)](c) sparks decline primarily by diffusion. Mitochondria also appear to have a direct effect on the properties of [Ca(2+)](c) sparks, because inhibition of mitochondrial Ca(2+) uptake results in an increase in the frequency and duration of [Ca(2+)](c) sparks. Thus, a short-lasting opening of a cluster of Ca(2+) release channels can yield activation of mitochondrial Ca(2+) uptake, and the competency of mitochondrial Ca(2+) handling may be an important determinant of cardiac excitability through local feedback control of elementary [Ca(2+)](c) signals.
Figures



References
-
- Parker I, Yao Y. Proc R Soc London Ser B. 1991;246:269–274. - PubMed
-
- Cheng H, Lederer W J, Cannell M B. Science. 1993;262:740–744. - PubMed
-
- Bootman M D, Berridge M J, Lipp P. Cell. 1997;91:367–373. - PubMed
-
- Berridge M J, Bootman M D, Lipp P. Nature (London) 1998;395:645–648. - PubMed
-
- Jaggar J H, Porter V A, Lederer W J, Nelson M T. Am J Physiol Cell Physiol. 2000;278:C235–C256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

