Analysis of MADS box protein-protein interactions in living plant cells
- PMID: 11854533
- PMCID: PMC122379
- DOI: 10.1073/pnas.042677699
Analysis of MADS box protein-protein interactions in living plant cells
Abstract
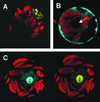
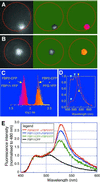
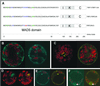
Over the last decade, the yeast two-hybrid system has become the tool to use for the identification of protein-protein interactions and recently, even complete interactomes were elucidated by this method. Nevertheless, it is an artificial system that is sensitive to errors resulting in the identification of false-positive and false-negative interactions. In this study, plant MADS box transcription factor interactions identified by yeast two-hybrid systems where studied in living plant cells by a technique based on fluorescence resonance energy transfer (FRET). Petunia MADS box proteins were fused to either cyan fluorescent protein or yellow fluorescent protein and transiently expressed in protoplasts followed by FRET-spectral imaging microscopy and FRET-fluorescence lifetime imaging microscopy to detect FRET and hence protein-protein interactions. All petunia MADS box heterodimers identified in yeast were confirmed in protoplasts. However, in contrast to the yeast two-hybrid results, homodimerization was demonstrated in plant cells for three petunia MADS box proteins. Heterodimers were identified between the ovule-specific MADS box protein FLORAL BINDING PROTEIN 11 and members of the petunia FLORAL BINDING PROTEIN 2 subfamily, which are also expressed in ovules, suggesting that these dimers play a role in ovule development. Furthermore, the role of dimerization in translocation of MADS box protein dimers to the nucleus is demonstrated, and the nuclear localization signal of MADS box proteins has been mapped to the N-terminal region of the MADS domain by means of mutant analyses.
Figures

References
-
- Fashena S J, Serebriiskii I, Golemis E A. Gene. 2000;250:1–14. - PubMed
-
- Uetz P, Giot L, Cagney G, Mansfield T A, Judson R S, Knight J R, Lockshon D, Narayan V, Srinivasan M, Pochart P, et al. Nature (London) 2000;403:623–627. - PubMed
-
- Stryer L. Annu Rev Biochem. 1978;47:819–846. - PubMed
-
- Wu P, Brand L. Anal Biochem. 1994;218:1–13. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

