Weekly cisplatin and daily oral etoposide is highly effective in platinum pretreated ovarian cancer
- PMID: 11857006
- PMCID: PMC2746539
- DOI: 10.1038/sj.bjc.6600002
Weekly cisplatin and daily oral etoposide is highly effective in platinum pretreated ovarian cancer
Abstract
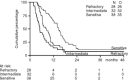
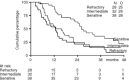
We investigated the potential of weekly cisplatin and daily oral etoposide followed by oral etoposide maintenance therapy in patients with platinum-refractory ovarium cancer. One hundred and seven patients were entered on the study, 98 patients completed the induction therapy consisting of cisplatin at either 50 or 70 mg m(-2) weekly for six administrations plus oral etoposide at a dose of 50 mg daily. Of these 98 patients, 38 had a platinum treatment-free interval of more than 12 months, 32 had an interval between 4 and 12 months, and 28 had progressed during or within 4 months after last platinum therapy. We assessed response rates and time to progression, and also response duration and survival. Analyses were done on the 98 evaluable patients. All 107 patients were considered evaluable for toxicity. Of the 38 patients with a treatment-free interval of more than 12 months, 92% responded, with 63% complete responses. The median progression-free survival in these patients was 14 months, and the median survival was 26 months. Of the 32 patients with an interval of 4-12 months, 91% responded, with 31% complete responses, a median progression-free interval of 8 and a median overall survival of 16 months. Of the 28 patients with platinum-refractory disease, 46% as yet responded, with 29% complete responses, median progression-free interval of 5 and an overall survival of 13 months. Haematologic and non-haematologic, particularly renal toxicity and neurotoxicity, were notably mild. We conclude that this intensive regimen of weekly cisplatin plus daily etoposide is highly effective and well tolerated in patients with ovarian cancer relapsing after conventional platinum-based combination chemotherapy, including patients who have progressed during or within 4 months after platinum treatment.
Figures
Comment in
-
Weekly platinum chemotherapy for recurrent ovarian cancer.Br J Cancer. 2002 Jan 7;86(1):2-4. doi: 10.1038/sj.bjc.6600062. Br J Cancer. 2002. PMID: 11857002 Free PMC article.
Similar articles
-
Phase II study of prolonged oral etoposide in patients with ovarian cancer refractory to or relapsing within 12 months after platinum-containing chemotherapy.Ann Oncol. 1994 Sep;5(7):656-7. doi: 10.1093/oxfordjournals.annonc.a058942. Ann Oncol. 1994. PMID: 7993845 Clinical Trial.
-
Weekly cisplatin and oral etoposide as treatment for relapsed epithelial ovarian cancer.Ann Oncol. 2001 Dec;12(12):1705-9. doi: 10.1023/a:1013558501425. Ann Oncol. 2001. PMID: 11843248
-
Weekly cisplatin with oral etoposide: a well-tolerated and highly effective regimen in relapsed ovarian cancer.Int J Gynecol Cancer. 2008 Mar-Apr;18(2):228-34. doi: 10.1111/j.1525-1438.2007.00994.x. Epub 2007 May 19. Int J Gynecol Cancer. 2008. PMID: 17511798
-
What is the role of dose-dense therapy?Int J Gynecol Cancer. 2005 Nov-Dec;15 Suppl 3:233-40. doi: 10.1111/j.1525-1438.2005.00432.x. Int J Gynecol Cancer. 2005. PMID: 16343238 Review.
-
[Carboplatin and etoposide combination for the treatment of recurrent epithelial ovarian cancer].Bull Cancer. 1996 Apr;83(4):315-23. Bull Cancer. 1996. PMID: 8680083 Review. French.
Cited by
-
Dose-dense chemotherapy improves mechanisms of antitumor immune response.Cancer Res. 2013 Jan 1;73(1):119-27. doi: 10.1158/0008-5472.CAN-12-2225. Epub 2012 Oct 29. Cancer Res. 2013. PMID: 23108141 Free PMC article. Clinical Trial.
-
Weekly cisplatin may reverse liver dysfunction and jaundice caused by diffuse liver metastases of solid tumors.Hepat Med. 2009 Oct 30;1:9-12. doi: 10.2147/hmer.s7574. eCollection 2009. Hepat Med. 2009. PMID: 24623997 Free PMC article.
-
Influence of dosing times on cisplatin-induced peripheral neuropathy in rats.BMC Cancer. 2016 Sep 27;16(1):756. doi: 10.1186/s12885-016-2777-0. BMC Cancer. 2016. PMID: 27678475 Free PMC article.
-
Weekly platinum chemotherapy for recurrent ovarian cancer.Br J Cancer. 2002 Jan 7;86(1):2-4. doi: 10.1038/sj.bjc.6600062. Br J Cancer. 2002. PMID: 11857002 Free PMC article.
-
A phase II trial of capecitabine (Xeloda) in recurrent ovarian cancer.Br J Cancer. 2003 Nov 17;89(10):1843-8. doi: 10.1038/sj.bjc.6601381. Br J Cancer. 2003. PMID: 14612890 Free PMC article. Clinical Trial.
References
-
- BerekJSBertelsenKdu BoisABradyMFCarmichaelJEisenhauerEAGoreMGrenmanSHamiltonTCHansenSWHarperPGHorvathGKayeSBLuckHJLundBMcGuireWPNeijtJPOzolsRFParmarMKPiccart-GebhartMJvan RijswijkRRosenbergPRustinGJSessaCWillemsePH1998Advanced epithelial ovarian cancer: 1998 consensus statement Ann Oncol 10S87S92
-
- BolisGScarfoneGLuchiniLFerrarisCZanaboniFPrestiMGiardinaGVillaAParazziniF1994Response to second-line weekly cisplatin chemotherapy in ovarian cancer previously treated and with a cisplatin or carboplatin-based regimen Eur J Cancer 30A17641768 - PubMed
-
- ChambersSKChambersJTKohornEISchwartzPE1987Etoposide plus cis-diamminedichloroplatinum as salvage therapy in advanced epithelial ovarian cancer Gynecol Oncol 27233240 - PubMed
-
- de WitRvan der BurgMELvan der GaastALogmansAStoterGVerweijJ1994Phase II study of prolonged oral etoposide in patients with ovarian cancer refractory to or relapsing within 12 months after platinum containing chemotherapy Ann Oncol 5656657 - PubMed
-
- EisenhauerEAten Bokkel HuininkWSwenertonKDGianniLMylesJvan der BurgMEKerrIVermorkenJBBuserKColomboN1994European-Canadian randomised trial of Taxol in relapsed ovarian cancer: High vs low and long vs short infusion J Clin Oncol 1226542666 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical