Roles of BCL-2 and caspase 3 in the adenosine A3 receptor-induced apoptosis
- PMID: 11859924
- PMCID: PMC5567771
- DOI: 10.1385/JMN:17:3:285
Roles of BCL-2 and caspase 3 in the adenosine A3 receptor-induced apoptosis
Abstract
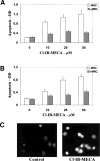
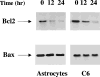
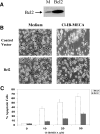
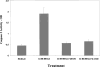
Selective A3 adenosine receptor agonists have been shown to induce apoptosis in a variety of cell types. In this study we examined the effects of adenosine receptor agonists selective for A1, A2A, or A3 receptors on the induction of apoptosis in primary cultures of rat astrocytes and in C6 glial cells. Treatment of the cells with the A3 receptor agonist Cl-IB-MECA (10 microM) induced apoptosis in both cell types. The effects of Cl-IB-MECA were partially antagonized by the A3 receptor-selective antagonist MRS 1191. In contrast, the A1 and A2A receptor agonists, CPA and CGS 21680, respectively, did not have significant effects on apoptosis in these cells. Cl-IB-MECA reduced the expression of endogenous Bcl-2, whereas it did not affect the expression of Bax. Overexpression of Bcl-2 in C6 cells abrogated the induction of apoptosis induced by the A3 agonist. Cl-IB-MECA also induced an increase in caspase 3 activity and caspase inhibitors decreased the apoptosis induced by the A3 agonist. These findings suggest that intense activation of the A3 receptor is pro-apoptotic in glial cells via bcl2 and caspase-3 dependent pathways.
Figures
References
-
- Brodie C, Bogi K, Acs P, Lorenzo PS, Baskin L, Blumberg PM. Protein kinase C delta (PKCdelta) inhibits the expression of glutamine synthetase in glial cells via the PKCdelta regulatory domain and its tyrosine phosphorylation. J Biol Chem. 1998b;273:30,713–30,718. - PubMed
-
- Brodie C, Weizman N, Katzoff A, Lustig S, Kobiler D. Astrocyte activation by Sindbis virus: expression of GFAP, cytokines, and adhesion molecules. Glia. 1997;19:275–285. - PubMed
-
- Ceruti S, Barbieri D, Franceschi C, Giammarioli AM, Rainaldi G, Malorni W, et al. Effects of adenosine A3 receptor agonists on astrocytes: induction of cell protection at low and cell death at high concentration. Drug Dev Res. 1996;37:177.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

