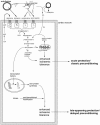
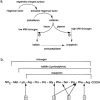
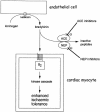
Role of bradykinin in preconditioning and protection of the ischaemic myocardium
- PMID: 11861312
- PMCID: PMC1573212
- DOI: 10.1038/sj.bjp.0704548
Role of bradykinin in preconditioning and protection of the ischaemic myocardium
Figures
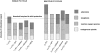
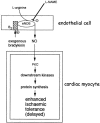
References
-
- AHMAD M., ZEITLIN I.J., PARRATT J.R. The release of kininase from rat isolated hearts during myocardial ischaemia. Immunopharmacology. 1996;33:299–300. - PubMed
-
- ANDERSON B., KHAPER N., DHALLA A.K., SINGAL P.K. Anti-free radical mechanisms in captopril protection against reperfusion injury in isolated rat hearts. Can. J. Cardiol. 1996;12:1099–1104. - PubMed
-
- ARAMORI I., ZENKOH J., MORIKAWA N., O'DONNELL N., ASANO M., NAKAMURA K., IWAMI M., KOJO H., NOTSU Y. Novel subtype-selective nonpeptide bradykinin receptor antagonists FR167344 and FR173657. Mol. Pharmacol. 1997;51:171–176. - PubMed
-
- ARMSTRONG S., GANOTE C.E. Adenosine receptor specificity in preconditioning of isolated rabbit cardiomyocytes: evidence of A3 receptor involvement. Cardiovasc. Res. 1994;28:1049–1056. - PubMed
-
- ASHER J.R., NAFTILAN A.J. Vasopeptidase inhibition: a new direction in cardiovascular treatment. Curr. Hypertens. Rep. 2000;2:384–391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

